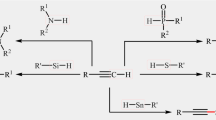
Abstract
Carbon–sulfur bond formation represents a key step in the synthesis of thioethers, which are a common structural motif in many pharmaceutically compounds. The direct cross-dehydrogenative coupling of C–H/S–H bonds has become a powerful tool for C–S bond formation. As these coupling reactions avoid pre-functionalization of the starting materials, they are more atom-economical, practical, and environmentally friendly than traditional cross-coupling reactions. In this review, we will highlight the most important developments in this novel and interesting research arena with the emphasis on the mechanistic aspects of the reactions. The review is divided into three major sections: (1) C(sp3)-H/S–H bonds coupling reactions; (2) C(sp2)-H/S–H bonds coupling reactions; and (3) C(sp)-H/S–H bonds coupling reactions.

















































Similar content being viewed by others
References
Feng M, Tang B, Liang S, Jiang X (2016) Curr Top Med Chem 16:1200
Scott KA, Njardarson JT (2018) Top Curr Chem 376:5
Abu-Yousef IA, Harpp DN (2003) Sulfur Rep 24:255
Wu Q, Zhao D, Qin X, Lan J, You J (2011) Chem Commun 47:9188
Chen M, Huang Z-T, Zheng Q-Y (2012) Chem Commun 48:11686
Zhao X, Lu X, Wei A, Jia X, Chen J, Lu K (2016) Tetrahedron Lett 57:5330
Jammi S, Barua P, Rout L, Saha P, Punniyamurthy T (2008) Tetrahedron Lett 49:1484
Reddy VP, Kumar AV, Swapna K, Rao KR (2009) Org Lett 11:1697
Lin Y-Y, Wang Y-J, Lin C–H, Cheng J-H, Lee C-F (2012) J Org Chem 77:6100
Herradura PS, Pendola KA, Guy RK (2000) Org Lett 2:2019
Itoh T, Mase T (2004) Org Lett 6:4587
Mispelaere-Canivet C, Spindler J-F, Perrio S, Beslin P (2005) Tetrahedron 61:5253
Beletskaya IP, Ananikov VP (2011) Chem Rev 111:1596
Savarin C, Srogl J, Liebeskind LS (2002) Org Lett 4:4309
Yeung CS, Dong VM (2011) Chem Rev 111:1215
Yuan J, Liu C, Lei A (2015) Chem Commun 51:1394
Chen T, Zhang J-S, Han L-B (2016) Dalton Trans 45:1843
Girard SA, Knauber T, Li CJ (2014) Angew Chem Int Ed 53:74
Lakshman MK, Vuram PK (2017) Chem Sci 8:5845
San Segundo M, Correa A (2018) Synthesis. https://doi.org/10.1055/s-0037-1610073
Arshadi S, Vessally E, Edjlali L, Hosseinzadeh-Khanmiri R, Ghorbani-Kalhor E (2017) Beilstein J Org Chem 13:625
Vessally E, Didehban K, Babazadeh M, Hosseinian A, Edjlali L (2017) J CO2 Util 21:480
Didehban K, Vessally E, Hosseinian A, Edjlali L, Khosroshahi ES (2018) RSC Adv 8:291
Vessally E, Didehban K, Mohammadi R, Hosseinian A, Babazadeh M (2018) J Sulfur Chem. https://doi.org/10.1080/17415993.2018.1436711
Vessally E, Mohammadi R, Hosseinian A, Didehban K, Edjlali L (2018) J Sulfur Chem. https://doi.org/10.1080/17415993.2018.1436712
Hosseinian A, Zare Fekri L, Monfared A, Vessally E, Nikpassand M (2018) J Sulfur Chem. https://doi.org/10.1080/17415993.2018.1471142
Yuan J, Ma X, Yi H, Liu C, Lei A (2014) Chem Commun 50:14386
Liao Y, Jiang P, Chen S, Qi H, Deng G-J (2013) Green Chem 15:3302
Chen Q, Huang Y, Wang X, Wen C, Yan X, Zeng J (2017) Tetrahedron Lett 58:3928
Chen Q, Wang X, Wen C, Huang Y, Yan X, Zeng J (2017) RSC Adv 7:39758
Chen X, Hao X-S, Goodhue CE, Yu J-Q (2006) J Am Chem Soc 128:6790
Iwasaki M, Iyanaga M, Tsuchiya Y, Nishimura Y, Li W, Li Z, Nishihara Y (2014) Chem Eur J 20:2459
Huang X, Chen Y, Zhen S, Song L, Gao M, Zhang P, Li H, Yuan B, Yang G (2018) J Org Chem 83:7331
Parumala SKR, Peddinti RK (2015) Green Chem 17:4068
Yan K, Yang D, Sun P, Wei W, Liu Y, Li G, Lu S, Wang H (2015) Tetrahedron Lett 56:4792
Yang D, Yan K, Wei W, Zhao J, Zhang M, Sheng X, Li G, Lu S, Wang H (2015) J Org Chem 80:6083
Xiao F, Tian J, Xing Q, Huang H, Deng GJ, Liu Y (2017) ChemistrySelect 2:428
Choudhury P, Roy B, Basu B (2017) Asian J Org Chem 6:1569
Huang Z, Zhang D, Qi X, Yan Z, Wang M, Yan H, Lei A (2016) Org Lett 18:2351
Liu W, Wang S, Jiang Y, He P, Zhang Q, Cao H (2015) Asian J Org Chem 4:312
Cao H, Chen L, Liu J, Cai H, Deng H, Chen G, Yan C, Chen Y (2015) RSC Adv 5:22356
Zheng Z, Qi D, Shi L (2015) Catal Commun 66:83
Hiebel M-A, Berteina-Raboin S (2015) Green Chem 17:937
Siddaraju Y, Prabhu KR (2016) J Org Chem 81:7838
Rahaman R, Das S, Barman P (2018) Green Chem 20:141
Maeda Y, Koyabu M, Nishimura T, Uemura S (2004) J Org Chem 69:7688
Yadav JS, Reddy BVS, Reddy YJ, Praneeth K (2009) Synthesis 2009:1520
Gensch T, Klauck FJ, Glorius F (2016) Angew Chem Int Ed 55:11287
Zhang X, Zhou X, Xiao H, Li X (2013) RSC Adv 3:22280
Liu Y, Zhang Y, Hu C, Wan J-P, Wen C (2014) RSC Adv 4:35528
Song S, Zhang Y, Yeerlan A, Zhu B, Liu J, Jiao N (2017) Angew Chem Int Ed 56:2487
Campbell JA, Broka CA, Gong L, Walker KA, Wang J-H (2004) Tetrahedron Lett 45:4073
Liu X, Cui H, Yang D, Dai S, Zhang G, Wei W, Wang H (2016) Catal Lett 146:1743
Yi S, Li M, Mo W, Hu X, Hu B, Sun N, Jin L, Shen Z (2016) Tetrahedron Lett 57:1912
Equbal D, Lavekar AG, Sinha AK (2016) Org Biomol Chem 14:6111
Ohkado R, Ishikawa T, Iida H (2018) Green Chem 20:984
Bai F, Zhang S, Wei L, Liu Y (2018) Asian J Org Chem 7:371
Guo W, Tan W, Zhao M, Tao K, Zheng L-Y, Wu Y, Chen D, Fan X-L (2017) RSC Adv 7:37739
Wang P, Tang S, Huang P, Lei A (2017) Angew Chem Int Ed 56:3009
Yadav J, Reddy BS, Reddy YJ (2007) Tetrahedron Lett 48:7034
Wu G, Wu J, Wu J, Wu L (2008) Synth Commun 38:1036
Choudhury P, Roy B, Basu B (2017) Asian J Org Chem 6:1569
Ranjit S, Lee R, Heryadi D, Shen C, Wu JE, Zhang P, Huang K-W, Liu X (2011) J Org Chem 76:8999
Zhou A-X, Liu X-Y, Yang K, Zhao S-C, Liang Y-M (2011) Org Biomol Chem 9:5456
Dai C, Xu Z, Huang F, Yu Z, Gao Y-F (2012) J Org Chem 77:4414
Inomata H, Toh A, Mitsui T, Fukuzawa S-i (2013) Tetrahedron Lett 54: 4729
Wang S, Liu W, Cai Z, Li S, Liu J, Wang A (2016) Synlett 27:2264
Yang D, Sun P, Wei W, Meng L, He L, Fang B, Jiang W, Wang H (2016) Org Chem Front 3:1457
Guo T, Wang H (2017) Synlett 28:1845
Zhao W, Xie P, Bian Z, Zhou A, Ge H, Niu B, Ding Y (2015) RSC Adv 5:59861
Yang Y, Dong W, Guo Y, Rioux RM (2013) Green Chem 15:3170
Fang Z, He W, Cai M, Lin Y, Zhao H (2015) Tetrahedron Lett 56:6463
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseinian, A., Ahmadi, S., Nasab, F.A.H. et al. Cross-Dehydrogenative C–H/S–H Coupling Reactions. Top Curr Chem (Z) 376, 39 (2018). https://doi.org/10.1007/s41061-018-0217-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-018-0217-0