Abstract
Purpose
Tubo-ovarian high-grade serous carcinoma is one of the aggressive ovarian tumors. Neoadjuvant chemotherapy has been used in managing the affected patients. The chemotherapy response score (CRS) system has been introduced to evaluate the patient’s response to neoadjuvant chemotherapy and their prognosis. The current study aimed to investigate the prognostic and clinicopathological significance of the CRS system in patients with tubo-ovarian high-grade serous carcinoma.
Methods
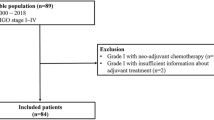
A total of 39 patients with tubo-ovarian high-grade serous carcinoma were included in the current study. We retrospectively investigated the progression-free survival (PFS), overall survival (OS), and their clinicopathological and surgical parameters. We also studied the Ki67 protein expression of included patients using immunohistochemistry (IHC). Besides, we investigated the significance of Ki67 expression in tubo-ovarian high-grade serous carcinoma development using the GSE73064 and GSE126308 datasets.
Results
Ki67 expression was not alerted in ascites, metastatic, and primary tubo-ovarian high-grade serous carcinoma. Also, Ki67 expression was not changed in the early tubo-ovarian high-grade serous carcinoma compared to late tubo-ovarian high-grade serous carcinoma. We showed that Ki67 protein expression was elevated in CRS1 patients with tubo-ovarian high-grade serous carcinoma compared to CRS3 patients. The bleeding amount and operation time were substantially lower in CRS3 patients compared to CRS1 patients, and there was a strong positive association between CRS3 and optimal resection. Furthermore, CRS3 patients had substantially improved PFS compared to CRS1 patients.
Conclusion
This study has highlighted the valuable role of the CRS system in determining the PFS and some clinicopathological features of tubo-ovarian high-grade serous carcinoma patients.



Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Kurman RJ, Shih I-M. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–47.
Singh N, McCluggage WG, Gilks CB. High-grade serous carcinoma of tubo-ovarian origin: recent developments. Histopathology. 2017;71(3):339–56.
Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–53.
Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. The Lancet. 2015;386(9990):249–57.
Böhm S, Faruqi A, Said I, Lockley M, Brockbank E, Jeyarajah A, et al. Chemotherapy response score: development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Clin Oncol. 2015;33(22):2457–63.
McCluggage WG, Judge MJ, Clarke BA, Davidson B, Gilks CB, Hollema H, et al. Data set for reporting of ovary, fallopian tube and primary peritoneal carcinoma: recommendations from the international collaboration on cancer reporting (ICCR). Mod Pathol. 2015;28(8):1101–22.
Coghlan E, Meniawy TM, Munro A, Bulsara M, Stewart CJ, Tan A, et al. Prognostic role of histological tumor regression in patients receiving neoadjuvant chemotherapy for high-grade serous tubo-ovarian carcinoma. Int J Gynecol Cancer. 2017;27(4):708–13.
Lee JY, Chung YS, Na K, Kim HM, Park CK, Nam EJ, et al. External validation of chemotherapy response score system for histopathological assessment of tumor regression after neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Gynecol Oncol. 2017;28(6): e73.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Mrouj K, Andrés-Sánchez N, Dubra G, Singh P, Sobecki M, Chahar D, et al. Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. Proc Natl Acad Sci. 2021;118(10): e2026507118.
Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 2015;11(3):1566–72.
Mahadevappa A, Krishna SM, Vimala MG. Diagnostic and prognostic significance of Ki-67 immunohistochemical expression in surface epithelial ovarian carcinoma. J Clin Diagn Res. 2017;11(2):Ec 08-ec12.
Ditzel HM, Strickland KC, Meserve EE, Stover E, Konstantinopoulos PA, Matulonis UA, et al. Assessment of a chemotherapy response score (CRS) system for tubo-ovarian high-grade serous carcinoma (HGSC). Int J Gynecol Pathol. 2019;38(3):230–40.
Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8(1):1093.
Chui MH, MomeniBoroujeni A, Mandelker D, Ladanyi M, Soslow RA. Characterization of TP53-wildtype tubo-ovarian high-grade serous carcinomas: rare exceptions to the binary classification of ovarian serous carcinoma. Mod Pathol. 2021;34(2):490–501.
Singh P, Kaushal V, Rai B, Rajwanshi A, Gupta N, Dey P, et al. The chemotherapy response score is a useful histological predictor of prognosis in high-grade serous carcinoma. Histopathology. 2018;72(4):619–25.
Coghlan E, Meniawy TM, Munro A, Bulsara M, Stewart CJ, Tan A, et al. Prognostic role of histological tumor regression in patients receiving neoadjuvant chemotherapy for high-grade serous tubo-ovarian carcinoma. International Journal of Gynecologic Cancer. 2017;27(4).
Santoro A, Angelico G, Piermattei A, Inzani F, Valente M, Arciuolo D, et al. Pathological chemotherapy response score in patients affected by high grade serous ovarian carcinoma: the prognostic role of omental and ovarian residual disease. Front Oncol. 2019;9:778.
Ali MA, Durrani N, Parveen K, Ahmad Z, Meenai FJ. Prognostic role of crs in high grade serous ovarian tumour patients receiving neoadjuvant chemotherapy in a tertiary care hospital of central india. Journal of Clinical & Diagnostic Research. 2022;16(5).
Lawson BC, Yang RK, Euscher ED, Ramalingam P, Malpica A. TP53 variant allele frequency correlates with the chemotherapy response score in ovarian/fallopian tube/peritoneal high-grade serous carcinoma. Hum Pathol. 2021;115:76–83.
Amadori D, Volpi A, Maltoni R, Nanni O, Amaducci L, Amadori A, et al. Cell proliferation as a predictor of response to chemotherapy in metastatic breast cancer: a prospective study. Breast Cancer Res Treat. 1997;43(1):7–14.
Rödel F, Zhou S, Győrffy B, Raab M, Sanhaji M, Mandal R, et al. The prognostic relevance of the proliferation markers Ki-67 and Plk1 in early-stage ovarian cancer patients with serous, low-grade carcinoma based on mRNA and protein expression. Front Oncol. 2020;10: 558932.
Zheng JN, Sun YF, Pei DS, Liu JJ, Ma TX, Han RF, et al. Treatment with vector-expressed small hairpin RNAs against Ki67 RNA-induced cell growth inhibition and apoptosis in human renal carcinoma cells. Acta Biochim Biophys Sin. 2006;38(4):254–61.
Hou Y-Y, Cao W-W, Li L, Li S-P, Liu T, Wan H-Y, et al. MicroRNA-519d targets MKi67 and suppresses cell growth in the hepatocellular carcinoma cell line QGY-7703. Cancer Lett. 2011;307(2):182–90.
Kaya R, Takanashi H, Nakajima A, Saito R, Yamaguchi N, Morimoto K, et al. Prognostic significance of Ki67 during neoadjuvant chemotherapy in primary unresectable ovarian cancer. J Obstet Gynaecol Res. 2021;47(11):3979–89.
Liu P, Sun YL, Du J, Hou XS, Meng H. CD105/Ki67 coexpression correlates with tumor progression and poor prognosis in epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22(4):586–92.
Acknowledgements
We appreciate all of the healthcare providers of Alzahra Hospital, a teaching and tertiary referral hospital affiliated with Tabriz University of Medical Sciences, Tabriz, Iran. The authors acknowledge the “Clinical Research Development Unit, Al-Zahra Hospital,” Tabriz University of Medical Sciences.
Funding
This work was supported by Tabriz University of Medical Sciences, Tabriz, Iran (Grant Number: 68828). P.M.G has received research support from Tabriz University of Medical Sciences, Tabriz, Iran.
Author information
Authors and Affiliations
Contributions
RD was involved in the investigation, methodology, data collection and analysis, writing—original draft, and writing—review and editing. AM contributed to the conceptualization, methodology, writing—original draft, and writing—review and editing. MSM assisted in the conceptualization and writing—review and editing. MJS was involved in the conceptualization, methodology, and writing—review and editing. VR contributed to the conceptualization, methodology, and writing—review and editing. MV assisted in the conceptualization, methodology, and writing—review and editing. ADT was involvd in the conceptualization, data analysis, and writing—review and editing. PMG contributed to the conceptualization, methodology, funding acquisition, supervision, writing—original draft, and writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1401.398).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Derogar, R., Mirzaei, A., Sayyah-Melli, M. et al. The Clinicopathological and Prognostic Values of Chemotherapy Response Score in Tubo-Ovarian High-Grade Serous Carcinoma. Indian J Gynecol Oncolog 22, 58 (2024). https://doi.org/10.1007/s40944-024-00812-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-024-00812-1