Abstract
Purpose
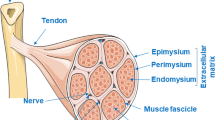
The extracellular matrix (ECM) is a complicated milieu consisting of structural and functional molecules secreted by the resident cells that provides an optimal microenvironmental niche for enhanced cell adhesion, growth, differentiation, and tissue formation and maturation. For decades, ECM bio-scaffolds prepared from decellularized tissues have been used to promote skeletal muscle regeneration; however, it was recently discovered that these decellularized ECM (dECM) materials can be further processed into hydrogels, thus expanding the potential applications of dECM materials in skeletal muscle regenerative engineering (SMRE). This review article highlights the recent advances in dECM-derived hydrogels toward skeletal muscle regeneration and repair.
Method
We screened articles in PubMed and bibliographic search using a combination of keywords. Relevant and high-cited articles were chosen for inclusion in this narrative review.
Results
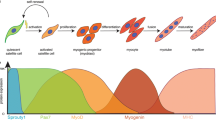
Here, we discuss the skeletal muscle ECM’s structure, function, and biochemical composition with emphasis on the role of the ECM during skeletal muscle embryogenesis, growth, development, and repair. Furthermore, we review various hydrogels used to promote skeletal muscle regeneration. We also review the current applications of dECM-derived hydrogels toward SMRE. Finally, we discuss the clinical translation potential of dECM-derived hydrogels for skeletal muscle regeneration and repair and their potential clinical considerations in the future.
Conclusion
Although much progress has been made in the field of dECM-derived hydrogels toward SMRE, it is still in its nascent stage. We believe improving and standardizing the methods of decellularization, lowering the immunogenicity of dECMs, and carrying out in vivo investigations in large animal models would advance their future clinical applications.
Lay Summary
Researchers have discovered an effective way to turn tissue materials into jelly-like substances known as extracellular matrix (ECM)-derived hydrogels. These ECM-derived hydrogels can help muscles heal better after serious injuries. They can be injected into gaps or used to guide muscle growth in the lab or body. This review article explains how these ECM-derived hydrogels are made and how they can be used to improve muscle healing. It also discusses their possible use in clinics and what needs to be considered before using them for medical treatments.






Similar content being viewed by others
References
Mulbauer GD, Matthew HWT. Biomimetic scaffolds in skeletal muscle regeneration. Discoveries. 7(1):e90, https://doi.org/10.15190/d.2019.3 (Craiova).
Csapo R, Gumpenberger M, Wessner B. Skeletal muscle extracellular matrix - what do we know about its composition, regulation, and physiological roles? A Narrative Review. Front Physiol. 2020;11:253. https://doi.org/10.3389/fphys.2020.00253.
Rowland LA, Bal NC, Periasamy M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev Camb Philos Soc. 2015;90(4):1279–97. https://doi.org/10.1111/brv.12157.
Baskin KK, Winders BR, Olson EN. Muscle as a “mediator“ of systemic metabolism. Cell Metab. 2015;21(2):237–48. https://doi.org/10.1016/j.cmet.2014.12.021.
Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8). https://doi.org/10.1038/nrendo.2012.49.
Kasukonis B, et al. Codelivery of infusion decellularized skeletal muscle with minced muscle autografts improved recovery from volumetric muscle loss injury in a rat model. Tissue Eng Part A. 2016;22(19–20):1151–63. https://doi.org/10.1089/ten.TEA.2016.0134.
Corona BT, Rivera JC, Owens JG, Wenke JC, Rathbone CR. Volumetric muscle loss leads to permanent disability following extremity trauma. J Rehabil Res Dev. 2015;52(7):785–92. https://doi.org/10.1682/JRRD.2014.07.0165.
Grogan BF, Hsu JR, STR Consortium. Volumetric muscle loss. JAAOS - J Am Acad Orthop Surg. 2011;19:S35.
Liu J, Saul D, Böker KO, Ernst J, Lehman W, Schilling AF. Current methods for skeletal muscle tissue repair and regeneration. BioMed Res Int. 2018. https://www.hindawi.com/journals/bmri/2018/1984879/. Accessed 04 Oct 2018.
Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819–29. https://doi.org/10.1016/S2213-8587(14)70034-8.
West SL, Lok CE, Jamal SA. Fracture risk assessment in chronic kidney disease, prospective testing under real world environments (FRACTURE): a prospective study. BMC Nephrol. 2010;11:17. https://doi.org/10.1186/1471-2369-11-17.
Bianchi B, Copelli C, Ferrari S, Ferri A, Sesenna E. Free flaps: outcomes and complications in head and neck reconstructions. J Craniomaxillofac Surg. 2009;37(8):438–42. https://doi.org/10.1016/j.jcms.2009.05.003.
Aguilar CA, et al. Multiscale analysis of a regenerative therapy for treatment of volumetric muscle loss injury. Cell Death Discov. 2018;4:33. https://doi.org/10.1038/s41420-018-0027-8.
Eckardt A, Fokas K. Microsurgical reconstruction in the head and neck region: an 18-year experience with 500 consecutive cases. J Craniomaxillofac Surg. 2003;31(4):197–201. https://doi.org/10.1016/s1010-5182(03)00039-8.
Stevanovic MV, Cuéllar VG, Ghiassi A, Sharpe F. Single-stage reconstruction of elbow flexion associated with massive soft-tissue defect using the latissimus dorsi muscle bipolar rotational transfer. Plast Reconstr Surg Glob Open. 2016;4(9):e1066. https://doi.org/10.1097/GOX.0000000000001066.
Barrera-Ochoa S, Collado-Delfa JM, Sallent A, Lluch A, Velez R. Free neurovascular latissimus dorsi muscle transplantation for reconstruction of hip abductors. Plast Reconstr Surg Glob Open. 2017;5(9):e1498. https://doi.org/10.1097/GOX.0000000000001498.
Laurencin CT, Khan Y. Regenerative engineering. Sci Transl Med. 2012;4(160):160ed9. https://doi.org/10.1126/scitranslmed.3004467.
Laurencin, C.T., & Khan, Y. (Eds.). (2013) Regenerative Engineering (1st ed.). CRC Press. https://doi.org/10.1201/b14925
Laurencin CT, Nair LS. The Quest toward limb regeneration: a regenerative engineering approach. Regen Biomater. 2016;3(2):123–5. https://doi.org/10.1093/rb/rbw002.
Mengsteab PY, Freeman J, Barajaa MA, Nair LS, Laurencin CT. Ligament regenerative engineering: braiding scalable and tunable bioengineered ligaments using a bench-top braiding machine. Regen Eng Transl Med. 2020. https://doi.org/10.1007/s40883-020-00178-8.
Barajaa MA, Nair LS, Laurencin CT. Bioinspired scaffold designs for regenerating musculoskeletal tissue interfaces. Regen Eng Transl Med. 2019. https://doi.org/10.1007/s40883-019-00132-3.
Daneshmandi L, Barajaa M, Tahmasbi Rad A, Sydlik SA, Laurencin CT. Graphene-based biomaterials for bone regenerative engineering: a comprehensive review of the field and considerations regarding biocompatibility and biodegradation. Adv Healthc Mater. 2021;10(1):2001414. https://doi.org/10.1002/adhm.202001414.
Barajaa MA, Nair LS, Laurencin CT. Robust phenotypic maintenance of limb cells during heterogeneous culture in a physiologically relevant polymeric-based constructed graft system. Sci Rep. 2020;10(1):11739. https://doi.org/10.1038/s41598-020-68658-z.
Ogueri KS, et al. In vivo evaluation of the regenerative capability of glycylglycine ethyl ester-substituted polyphosphazene and poly(lactic-co-glycolic acid) blends: a rabbit critical-sized bone defect model. ACS Biomater Sci Eng. 2021;7(4):1564–72. https://doi.org/10.1021/acsbiomaterials.0c01650.
Seyedsalehi A, Daneshmandi L, Barajaa M, Riordan J, Laurencin CT. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci Rep. 2020;10(1):22210. https://doi.org/10.1038/s41598-020-78977-w.
Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17(Suppl 4):467–79. https://doi.org/10.1007/s00586-008-0745-3.
Tang X, Daneshmandi L, Awale G, Nair LS, Laurencin CT. Skeletal muscle regenerative engineering. Regen Eng Transl Med. 2019;5(3):233–51. https://doi.org/10.1007/s40883-019-00102-9.
Lev R, Seliktar D. Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J R Soc Interface. 2018;15(138):20170380. https://doi.org/10.1098/rsif.2017.0380.
Fischer KM, et al. Hydrogels for skeletal muscle regeneration. Regen Eng Transl Med. 2021;7(3):353–61. https://doi.org/10.1007/s40883-019-00146-x.
Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 2017;49:1–15. https://doi.org/10.1016/j.actbio.2016.11.068.
Boso D, Maghin E, Carraro E, Giagante M, Pavan P, Piccoli M. Extracellular matrix-derived hydrogels as biomaterial for different skeletal muscle tissue replacements. Materials. 2020;13(11):2483. https://doi.org/10.3390/ma13112483. (Basel).
Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23(8 Suppl 1):S20-23. https://doi.org/10.1097/IJG.0000000000000108.
Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014;163(4):268–85. https://doi.org/10.1016/j.trsl.2013.11.003.
Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318–31. https://doi.org/10.1002/mus.22094.
The importance of extracellular matrix in skeletal muscle development and function | IntechOpen. https://www.intechopen.com/chapters/49985. Accessed 06 Jun 2022.
Purslow PP. The structure and role of intramuscular connective tissue in muscle function. Front Physiol. 2020;11:495. https://doi.org/10.3389/fphys.2020.00495.
Passerieux E, et al. Structural organization of the perimysium in bovine skeletal muscle: Junctional plates and associated intracellular subdomains. J Struct Biol. 2006;154(2):206–16. https://doi.org/10.1016/j.jsb.2006.01.002.
Stecco C, Hammer W, Vleeming A, De Caro R. 3 - Deep Fasciae. In Functional Atlas of the Human Fascial System, C. Stecco, W. Hammer, A. Vleeming, and R. De Caro, Eds., Churchill Livingstone; 2015. pp. 51–102. https://doi.org/10.1016/B978-0-7020-4430-4.00003-8.
Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278(15):12601–4. https://doi.org/10.1074/jbc.R200027200.
Khalilgharibi N, Mao Y. To form and function: on the role of basement membrane mechanics in tissue development, homeostasis and disease. Open Biol. 2021;11(2):200360. https://doi.org/10.1098/rsob.200360.
Holmberg J, Durbeej M. Laminin-211 in skeletal muscle function. Cell Adh Migr. 2013;7(1):111–21. https://doi.org/10.4161/cam.22618.
Kjær M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84(2):649–98. https://doi.org/10.1152/physrev.00031.2003.
Janson IA, Putnam AJ. Extracellular matrix elasticity and topography: material-based cues that affect cell function via conserved mechanisms. J Biomed Mater Res A. 2015;103(3):1246–58. https://doi.org/10.1002/jbm.a.35254.
Grzelkowska-Kowalczyk K. The importance of extracellular matrix in skeletal muscle development and function. IntechOpen. 2016. https://doi.org/10.5772/62230.
Thorsteinsdóttir S, Deries M, Cachaço AS, Bajanca F. The extracellular matrix dimension of skeletal muscle development. Dev Biol. 2011;354(2):191–207. https://doi.org/10.1016/j.ydbio.2011.03.015.
Takala TE, Virtanen P. Biochemical composition of muscle extracellular matrix: the effect of loading. Scand J Med Sci Sports. 2000;10(6):321–5. https://doi.org/10.1034/j.1600-0838.2000.010006321.x.
Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. https://doi.org/10.1007/978-94-007-7893-1_3.
Duance VC, Restall DJ, Beard H, Bourne FJ, Bailey AJ. The location of three collagen types in skeletal muscle. FEBS Lett. 1977;79(2):248–52. https://doi.org/10.1016/0014-5793(77)80797-7.
Light N, Champion AE. Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem J. 1984;219(3):1017–26. https://doi.org/10.1042/bj2191017.
McKee TJ, Perlman G, Morris M, Komarova SV. Extracellular matrix composition of connective tissues: a systematic review and meta-analysis. Sci Rep. 2019;9(1):10542. https://doi.org/10.1038/s41598-019-46896-0.
Kovanen V. Intramuscular extracellular matrix: complex environment of muscle cells. Exerc Sport Sci Rev. 2002;30(1):20–5. https://doi.org/10.1097/00003677-200201000-00005.
Sand JMB, Genovese F, Gudmann NS, Karsdal MA. Chapter 4 - Type IV collagen. In Biochemistry of Collagens, Laminins and Elastin (Second Edition), M. A. Karsdal, Ed., Academic Press; 2019. pp. 37–49. https://doi.org/10.1016/B978-0-12-817068-7.00004-5.
Leeming DJ, Karsdal MA. Chapter 5 - Type V collagen. In Biochemistry of Collagens, Laminins and Elastin (Second Edition), M. A. Karsdal, Ed., Academic Press; 2019. pp. 51–57. https://doi.org/10.1016/B978-0-12-817068-7.00005-7.
Bönnemann CG. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol. 2011;7(7):379–90. https://doi.org/10.1038/nrneurol.2011.81.
Sabatelli P, et al. Expression of collagen VI α5 and α6 chains in human muscle and in Duchenne muscular dystrophy-related muscle fibrosis. Matrix Biol. 2012;31(3):187–96. https://doi.org/10.1016/j.matbio.2011.12.003.
Cescon M, Gattazzo F, Chen P, Bonaldo P. Collagen VI at a glance. J Cell Sci. 2015;128(19):3525–31. https://doi.org/10.1242/jcs.169748.
Chiquet M, Birk DE, Bönnemann CG, Koch M. Collagen XII: protecting bone and muscle integrity by organizing collagen fibrils. Int J Biochem Cell Biol. 2014;53:51–4. https://doi.org/10.1016/j.biocel.2014.04.020.
Koch M, et al. A novel marker of tissue junctions, collagen XXII. J Biol Chem. 2004;279(21):22514–21. https://doi.org/10.1074/jbc.M400536200.
Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239(1):79–94. https://doi.org/10.1006/dbio.2001.0416.
Handley CJ, Samiric T, Ilic MZ. Structure, metabolism, and tissue roles of chondroitin sulfate proteoglycans. Adv Pharmacol. 2006;53:219–32. https://doi.org/10.1016/S1054-3589(05)53010-2.
Brandan E. Proteoglycans in skeletal muscle. Braz J Med Biol Res. 1994;27(9):2109–16.
Kölbel H, Hathazi D, Jennings M, Horvath R, Roos A, Schara U. Identification of candidate protein markers in skeletal muscle of laminin-211-deficient CMD type 1A-patients. Front Neurol. 2019;10. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fneur.2019.00470. Accessed 06 Jun 2022.
Boppart MD, Mahmassani ZS. Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2019;317(4):C629–41. https://doi.org/10.1152/ajpcell.00009.2019.
Franchi MV, Reeves ND, Narici MV. Skeletal muscle remodeling in response to eccentric vs. concentric loading: morphological, molecular, and metabolic adaptations. Front Physiol. 2017;8. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fphys.2017.00447. Accessed 23 Jun 2022.
Guérin CW, Holland PC. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Dev Dyn. 1995;202(1):91–9. https://doi.org/10.1002/aja.1002020109.
Nishimura T, Nakamura K, Kishioka Y, Kato-Mori Y, Wakamatsu J, Hattori A. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. J Muscle Res Cell Motil. 2008;29(1):37–44. https://doi.org/10.1007/s10974-008-9140-2.
Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res. 2015;56(1):1–8. https://doi.org/10.3109/03008207.2014.947369.
Jakobsen JR, Mackey AL, Knudsen AB, Koch M, Kjaer M, Krogsgaard MR. Composition and adaptation of human myotendinous junction and neighboring muscle fibers to heavy resistance training. Scand J Med Sci Sports. 2017;27(12):1547–59. https://doi.org/10.1111/sms.12794.
Härönen H, et al. Collagen XIII secures pre- and postsynaptic integrity of the neuromuscular synapse. Hum Mol Genet. 2017;26(11):2076–90. https://doi.org/10.1093/hmg/ddx101.
Heikkinen A, Härönen H, Norman O, Pihlajaniemi T. Collagen XIII and other ECM components in the assembly and disease of the neuromuscular junction. Anat Rec. 2020;303(6):1653–63. https://doi.org/10.1002/ar.24092. (Hoboken).
Eklund L, et al. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc Natl Acad Sci. 2001;98(3):1194–9. https://doi.org/10.1073/pnas.98.3.1194.
Guillon E, Bretaud S, Ruggiero F. Slow muscle precursors lay down a collagen XV matrix fingerprint to guide motor axon navigation. J Neurosci. 2016;36(9):2663–76. https://doi.org/10.1523/JNEUROSCI.2847-15.2016.
Heljasvaara R, Aikio M, Ruotsalainen H, Pihlajaniemi T. Collagen XVIII in tissue homeostasis and dysregulation - lessons learned from model organisms and human patients. Matrix Biol. 2017;57–58:55–75. https://doi.org/10.1016/j.matbio.2016.10.002.
Khaleduzzaman M, Sumiyoshi H, Ueki Y, Inoguchi K, Ninomiya Y, Yoshioka H. Structure of the human type XIX collagen (COL19A1) gene, which suggests it has arisen from an ancestor gene of the FACIT family. Genomics. 1997;45(2):304–12. https://doi.org/10.1006/geno.1997.4921.
Sumiyoshi H, Laub F, Yoshioka H, Ramirez F. Embryonic expression of type XIX collagen is transient and confined to muscle cells. Dev Dyn. 2001;220(2):155–62. https://doi.org/10.1002/1097-0177(2000)9999:9999%3c::AID-DVDY1099%3e3.0.CO;2-W.
Charvet B, et al. Knockdown of col22a1 gene in zebrafish induces a muscular dystrophy by disruption of the myotendinous junction. Development. 2013;140(22):4602–13. https://doi.org/10.1242/dev.096024.
Pisconti A, Bernet JD, Olwin BB. Syndecans in skeletal muscle development, regeneration and homeostasis. Muscles Ligaments Tendons J. 2012;2(1):1–9.
Decorin, a growth hormone-regulated protein in humans in: European Journal of Endocrinology. 2018;178(2). https://eje.bioscientifica.com/view/journals/eje/178/2/EJE-17-0844.xml. Accessed 06 Jun 2022.
Yamashita Y, et al. Perlecan, a heparan sulfate proteoglycan, regulates systemic metabolism with dynamic changes in adipose tissue and skeletal muscle. Sci Rep. 2018;8:7766. https://doi.org/10.1038/s41598-018-25635-x.
Nastase MV, Young MF, Schaefer L. Biglycan. J Histochem Cytochem. 2012;60(12):963–75. https://doi.org/10.1369/0022155412456380.
Werle MJ. Neuromuscular junction (NMJ): postsynaptic basal lamina. In Encyclopedia of Neuroscience, L. R. Squire, Ed., Oxford: Academic Press; 2009. pp 595–600. https://doi.org/10.1016/B978-008045046-9.01285-7.
Ren X, Zhao M, Lash B, Martino MM, Julier Z. Growth factor engineering strategies for regenerative medicine applications. Front Bioeng Biotechnol. 2020;7. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fbioe.2019.00469. Accessed 23 Jun 2022.
Syverud BC, VanDusen KW, Larkin LM. Growth factors for skeletal muscle tissue engineering. Cells Tissues Organs. 2016;202(3–4):169–79. https://doi.org/10.1159/000444671.
Wilgus TA. Growth factor–extracellular matrix interactions regulate wound repair. Adv Wound Care. 2012;1(6):249–54. https://doi.org/10.1089/wound.2011.0344. (New Rochelle).
Liu D, Black BL, Derynck R. TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15(22):2950–66. https://doi.org/10.1101/gad.925901.
TGFB3 gene: MedlinePlus Genetics. https://medlineplus.gov/genetics/gene/tgfb3/. Accessed 05 Jun 2022.
Arsic N, et al. Vascular endothelial growth factor stimulates skeletal muscle regeneration in Vivo. Mol Ther. 2004;10(5):844–54. https://doi.org/10.1016/j.ymthe.2004.08.007.
Piñol-Jurado P, et al. Platelet-derived growth factor bb influences muscle regeneration in Duchenne muscle dystrophy. Am J Pathol. 2017;187(8):1814–27. https://doi.org/10.1016/j.ajpath.2017.04.011.
Barclay RD, Burd NA, Tyler C, Tillin NA, Mackenzie RW. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front Nutr. 2019;6. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fnut.2019.00146. Accessed 05 Jun 2022.
Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1(1):4. https://doi.org/10.1186/2044-5040-1-4.
D’Andrea P, Sciancalepore M, Veltruska K, Lorenzon P, Bandiera A. Epidermal growth factor – based adhesion substrates elicit myoblast scattering, proliferation, differentiation and promote satellite cell myogenic activation. Biochim Biophys Acta (BBA) – Mol Cell Res. 2019;1866(3):504–517. https://doi.org/10.1016/j.bbamcr.2018.10.012.
Jia W, et al. Effects of fasting on the expression pattern of FGFs in different skeletal muscle fibre types and sexes in mice. Biol Sex Differ. 2020;11(1):9. https://doi.org/10.1186/s13293-020-00287-7.
Choi W, Lee J, Lee J, Lee SH, Kim S. Hepatocyte growth factor regulates macrophage transition to the M2 phenotype and promotes murine skeletal muscle regeneration. Front Physiol. 2019;10. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fphys.2019.00914. Accessed 05 Jun 2022.
Yamaguchi A, Sakuma K, Fujikawa T, Morita I. Expression of specific IGFBPs is associated with those of the proliferating and differentiating markers in regenerating rat plantaris muscle. J Physiol Sci. 2013;63(1). https://doi.org/10.1007/s12576-012-0227-6.
Pearse RV, Scherz PJ, Campbell JK, Tabin CJ. A cellular lineage analysis of the chick limb bud. Dev Biol. 2007;310(2):388–400. https://doi.org/10.1016/j.ydbio.2007.08.002.
Nowicki JL, Takimoto R, Burke AC. The lateral somitic frontier: dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mech Dev. 2003;120(2):227–40. https://doi.org/10.1016/s0925-4773(02)00415-x.
Olsson L, Falck P, Lopez K, Cobb J, Hanken J. Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Dev Biol. 2001;237(2):354–67. https://doi.org/10.1006/dbio.2001.0377.
Dietrich S, et al. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126(8):1621–9. https://doi.org/10.1242/dev.126.8.1621.
Swartz ME, Eberhart J, Pasquale EB, Krull CE. EphA4/ephrin-A5 interactions in muscle precursor cell migration in the avian forelimb. Development. 2001;128(23):4669–80. https://doi.org/10.1242/dev.128.23.4669.
Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell. 2003;5(6):937–44. https://doi.org/10.1016/s1534-5807(03)00360-5.
Hasson P, et al. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev Cell. 2010;18(1):148–56. https://doi.org/10.1016/j.devcel.2009.11.013.
Iwata J, Suzuki A, Pelikan RC, Ho T-V, Chai Y. Noncanonical transforming growth factor β (TGFβ) signaling in cranial neural crest cells causes tongue muscle developmental defects. J Biol Chem. 2013;288(41):29760–70. https://doi.org/10.1074/jbc.M113.493551.
Vallecillo-García P, et al. Odd skipped-related 1 identifies a population of embryonic fibro-adipogenic progenitors regulating myogenesis during limb development. Nat Commun. 2017;8(1):1218. https://doi.org/10.1038/s41467-017-01120-3.
Musarò A. The basis of muscle regeneration. Advances in Biology. 2014;2014:e612471. https://doi.org/10.1155/2014/612471.
Almada AE, Wagers AJ. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat Rev Mol Cell Biol. 2016;17(5):267–79. https://doi.org/10.1038/nrm.2016.7.
Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275(2):375–88. https://doi.org/10.1016/j.ydbio.2004.08.015.
Wilschut KJ, Haagsman HP, Roelen BAJ. Extracellular matrix components direct porcine muscle stem cell behavior. Exp Cell Res. 2010;316(3):341–52. https://doi.org/10.1016/j.yexcr.2009.10.014.
Grefte S, Vullinghs S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed Mater. 2012;7(5):055004. https://doi.org/10.1088/1748-6041/7/5/055004.
Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–81. https://doi.org/10.1126/science.1191035.
Cosgrove BD, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20(3):255–64. https://doi.org/10.1038/nm.3464.
Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12(1):75–87. https://doi.org/10.1016/j.stem.2012.09.015.
Urciuolo A, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964. https://doi.org/10.1038/ncomms2964.
Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2(1):50–9. https://doi.org/10.1016/j.stem.2007.10.006.
Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54(11):1177–91. https://doi.org/10.1369/jhc.6R6995.2006.
Boonen KJM, Rosaria-Chak KY, Baaijens FPT, van der Schaft DWJ, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol. 2009;296(6):C1338-1345. https://doi.org/10.1152/ajpcell.00015.2009.
Rønning SB, Pedersen ME, Andersen PV, Hollung K. The combination of glycosaminoglycans and fibrous proteins improves cell proliferation and early differentiation of bovine primary skeletal muscle cells. Differentiation. 2013;86(1–2):13–22. https://doi.org/10.1016/j.diff.2013.06.006.
Gutiérrez J, Brandan E. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol. 2010;30(7):1634–49. https://doi.org/10.1128/MCB.01164-09.
Rayagiri SS, et al. Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat Commun. 2018;9(1):1075. https://doi.org/10.1038/s41467-018-03425-3.
Baghdadi MB, et al. Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche. Nature. 2018;557(7707):714–8. https://doi.org/10.1038/s41586-018-0144-9.
Chargé SBP, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–38. https://doi.org/10.1152/physrev.00019.2003.
Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995;27(7):1022–32. https://doi.org/10.1249/00005768-199507000-00011.
Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41(4):457–65.
Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30(11):511–28. https://doi.org/10.1093/intimm/dxy054.
Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5. https://doi.org/10.1083/jcb.9.2.493.
Skuk D, Goulet M, Tremblay JP. Transplanted myoblasts can migrate several millimeters to fuse with damaged myofibers in nonhuman primate skeletal muscle. J Neuropathol Exp Neurol. 2011;70(9):770–8. https://doi.org/10.1097/NEN.0b013e31822a6baa.
Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (1985). 2001;91(2):534–51. https://doi.org/10.1152/jappl.2001.91.2.534.
Kääriäinen M, Järvinen T, Järvinen M, Rantanen J, Kalimo H. Relation between myofibers and connective tissue during muscle injury repair. Scand J Med Sci Sports. 2000;10(6):332–7. https://doi.org/10.1034/j.1600-0838.2000.010006332.x.
Mann CJ, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1(1):21. https://doi.org/10.1186/2044-5040-1-21.
Ambrosi D, et al. Growth and remodelling of living tissues: perspectives, challenges and opportunities. J R Soc Interface. 2019;16(157):20190233. https://doi.org/10.1098/rsif.2019.0233.
Bryan BA, et al. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. MBoC. 2008;19(3):994–1006. https://doi.org/10.1091/mbc.e07-09-0856.
Mofarrahi M, et al. Angiopoietin-1 enhances skeletal muscle regeneration in mice. Am J Physiol Regul Integr Comp Physiol. 2015;308(7):R576-589. https://doi.org/10.1152/ajpregu.00267.2014.
Christov C, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18(4):1397–409. https://doi.org/10.1091/mbc.e06-08-0693.
Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194(1):114–28. https://doi.org/10.1006/dbio.1997.8803.
Forcina L, Miano C, Pelosi L, Musarò A. An overview about the biology of skeletal muscle satellite cells. Curr Genomics. 2019;20(1):24–37. https://doi.org/10.2174/1389202920666190116094736.
Bahram M, Mohseni N, Moghtader M. An introduction to hydrogels and some recent applications. IntechOpen. 2016. https://doi.org/10.5772/64301.
Mantha S, et al. Smart hydrogels in tissue engineering and regenerative medicine. Materials. 2019;12(20):3323. https://doi.org/10.3390/ma12203323. (Basel).
Pollot BE, Rathbone CR, Wenke JC, Guda T. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J Biomed Mater Res B Appl Biomater. 2018;106(2):672–9. https://doi.org/10.1002/jbm.b.33859.
Saroia J, Yanen W, Wei Q, Zhang K, Lu T, Zhang B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des Manuf. 2018;1(4):265–79. https://doi.org/10.1007/s42242-018-0029-7.
Lin K, Zhang D, Macedo MH, Cui W, Sarmento B, Shen G. Advanced collagen-based biomaterials for regenerative biomedicine. Adv Func Mater. 2019;29(3):1804943. https://doi.org/10.1002/adfm.201804943.
Kim JK, Kim HJ, Chung J-Y, Lee J-H, Young S-B, Kim Y-H. Natural and synthetic biomaterials for controlled drug delivery. Arch Pharm Res. 2014;37(1):60–8. https://doi.org/10.1007/s12272-013-0280-6.
Dietrich M, et al. Fibrin-based tissue engineering: comparison of different methods of autologous fibrinogen isolation. Tissue Eng Part C Methods. 2013;19(3):216–26. https://doi.org/10.1089/ten.TEC.2011.0473.
Catoira MC, Fusaro L, Di Francesco D, Ramella M, Boccafoschi F. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci: Mater Med. 2019;30(10):115. https://doi.org/10.1007/s10856-019-6318-7.
Rastogi P, Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4):042001. https://doi.org/10.1088/1758-5090/ab331e.
Li H, Tan C, Li L. Review of 3D printable hydrogels and constructs. Mater Des. 2018;159:20–38. https://doi.org/10.1016/j.matdes.2018.08.023.
Bitas D, Samanidou V, Kabir A, Lucena R, Cárdenas S. 9 - Membrane sorptive phases. In Analytical Sample Preparation With Nano- and Other High-Performance Materials, R. Lucena and S. Cárdenas, Eds., Elsevier; 2021. pp. 199–228. https://doi.org/10.1016/B978-0-12-822139-6.00015-8.
Roberts JJ, Martens PJ. 9 - Engineering biosynthetic cell encapsulation systems. In Biosynthetic Polymers for Medical Applications, L. Poole-Warren, P. Martens, and R. Green, Eds., in Woodhead Publishing Series in Biomaterials. Woodhead Publishing; 2016. pp. 205–239. https://doi.org/10.1016/B978-1-78242-105-4.00009-2.
Stringer R. ELECTROPHORESIS | Overview. In Encyclopedia of Analytical Science (Second Edition), P. Worsfold, A. Townshend, and C. Poole, Eds., Oxford: Elsevier; 2005. pp. 356–363. https://doi.org/10.1016/B0-12-369397-7/00120-5.
Matthias N, et al. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs). Stem Cell Res. 2018;27:65–73. https://doi.org/10.1016/j.scr.2018.01.008.
Thorrez L, DiSano K, Shansky J, Vandenburgh H. Engineering of human skeletal muscle with an autologous deposited extracellular matrix. Front Physiol. 2018;9. [Online]. Available: https://www.frontiersin.org/article/https://doi.org/10.3389/fphys.2018.01076. Accessed 24 Jun 2022.
Marcinczyk M, Elmashhady H, Talovic M, Dunn A, Bugis F, Garg K. Laminin-111 enriched fibrin hydrogels for skeletal muscle regeneration. Biomaterials. 2017;141:233–42. https://doi.org/10.1016/j.biomaterials.2017.07.003.
Cappello V, et al. Ultrastructural characterization of the lower motor system in a mouse model of Krabbe disease. Sci Rep. 2016;6(1):1. https://doi.org/10.1038/s41598-016-0001-8.
Jaipan P, Nguyen A, Narayan RJ. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017;7(3):416–26. https://doi.org/10.1557/mrc.2017.92.
Gyles DA, Castro LD, Silva JOC, Ribeiro-Costa RM. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur Polymer J. 2017;88:373–92. https://doi.org/10.1016/j.eurpolymj.2017.01.027.
Akhtar MF, Hanif M, Ranjha NM. Methods of synthesis of hydrogels … a review. Saudi Pharmac J. 2016;24(5):554–9. https://doi.org/10.1016/j.jsps.2015.03.022.
Yang G, et al. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ. 2016;4:e2497. https://doi.org/10.7717/peerj.2497.
Kirchmajer DM, Watson CA, Ranson M, Panhuis M. Gelapin, a degradable genipin cross-linked gelatin hydrogel. RSC Adv. 2012;3(4):1073–81. https://doi.org/10.1039/C2RA22859A.
Gupta D, Santoso JW, McCain ML. Characterization of gelatin hydrogels cross-linked with microbial transglutaminase as engineered skeletal muscle substrates. Bioengineering. 2021;8(1):6. https://doi.org/10.3390/bioengineering8010006. (Basel).
Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283(5):C1557–65. https://doi.org/10.1152/ajpcell.00595.2001.
Chiron S, et al. Complex interactions between human myoblasts and the surrounding 3D fibrin-based matrix. PLoS ONE. 2012;7(4):e36173. https://doi.org/10.1371/journal.pone.0036173.
Mahdy MAA. Skeletal muscle fibrosis: an overview. Cell Tissue Res. 2019;375(3):575–88. https://doi.org/10.1007/s00441-018-2955-2.
Brown AC, Barker TH. Fibrin-based biomaterials: modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014;10(4):1502–14. https://doi.org/10.1016/j.actbio.2013.09.008.
Volpi M, Paradiso A, Costantini M, Świȩszkowski W. Hydrogel-based fiber biofabrication techniques for skeletal muscle tissue engineering. ACS Biomater Sci Eng. 2022;8(2):379–405. https://doi.org/10.1021/acsbiomaterials.1c01145.
Solorio L, Zwolinski C, Lund AW, Farrell MJ, Stegemann JP. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J Tissue Eng Regen Med. 2010;4(7):514–23. https://doi.org/10.1002/term.267.
Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8(5):607–26. https://doi.org/10.1586/erd.11.27.
Jia J, et al. Development of peptide-functionalized synthetic hydrogel microarrays for stem cell and tissue engineering applications. Acta Biomater. 2016;45:110–20. https://doi.org/10.1016/j.actbio.2016.09.006.
Oh SH, Lee JH. Hydrophilization of synthetic biodegradable polymer scaffolds for improved cell/tissue compatibility. Biomed Mater. 2013;8(1):014101. https://doi.org/10.1088/1748-6041/8/1/014101.
Villa C, et al. P(NIPAAM-co-HEMA) thermoresponsive hydrogels: an alternative approach for muscle cell sheet engineering. J Tissue Eng Regen Med. 2017;11(1):187–96. https://doi.org/10.1002/term.1898.
Browe DP, et al. Characterization and optimization of actuating poly(ethylene glycol) diacrylate/acrylic acid hydrogels as artificial muscles. Polymer. 2017;117:331–41. https://doi.org/10.1016/j.polymer.2017.04.044. (Guildf).
Cha SH, Lee HJ, Koh W-G. Study of myoblast differentiation using multi-dimensional scaffolds consisting of nano and micropatterns. Biomater Res. 2017;21(1):1. https://doi.org/10.1186/s40824-016-0087-x.
Vannozzi L, Yasa IC, Ceylan H, Menciassi A, Ricotti L, Sitti M. Self-folded hydrogel tubes for implantable muscular tissue scaffolds. Macromol Biosci. 2018;18(4):e1700377. https://doi.org/10.1002/mabi.201700377.
Xu Y, et al. Regulating myogenic differentiation of mesenchymal stem cells using thermosensitive hydrogels. Acta Biomater. 2015;26:23–33. https://doi.org/10.1016/j.actbio.2015.08.010.
Hosseinzadeh S, Rezayat SM, Giaseddin A, Aliyan A, Soleimani M. Microfluidic system for synthesis of nanofibrous conductive hydrogel and muscle differentiation. J Biomater Appl. 2018;32(7):853–61. https://doi.org/10.1177/0885328217744377.
McKeon-Fischer KD, Flagg DH, Freeman JW. Coaxial electrospun poly(ε-caprolactone), multiwalled carbon nanotubes, and polyacrylic acid/polyvinyl alcohol scaffold for skeletal muscle tissue engineering. J Biomed Mater Res A. 2011;99(3):493–9. https://doi.org/10.1002/jbm.a.33116.
Gaharwar AK, Peppas NA, Khademhosseini A. Nanocomposite hydrogels for biomedical applications. Biotechnol Bioeng. 2014;111(3):441–53. https://doi.org/10.1002/bit.25160.
Hwang JH, et al. Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration. Biomaterials. 2013;34(25):6037–45. https://doi.org/10.1016/j.biomaterials.2013.04.049.
Mulyasasmita W, et al. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J Control Release. 2014;191:71–81. https://doi.org/10.1016/j.jconrel.2014.05.015.
Fuoco C, et al. 3D hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering. Front Physiol. 2014;5:203. https://doi.org/10.3389/fphys.2014.00203.
In vivo generation of a mature and functional artificial skeletal muscle. EMBO Mol Med. 2015;7(4):411–422. https://doi.org/10.15252/emmm.201404062.
Rich MH, et al. Water–hydrogel binding affinity modulates freeze-drying-induced micropore architecture and skeletal myotube formation. Biomacromol. 2015;16(8):2255–64. https://doi.org/10.1021/acs.biomac.5b00652.
Urciuolo A, De Coppi P. Decellularized tissue for muscle regeneration. Int J Mol Sci. 2018;19(8):2392. https://doi.org/10.3390/ijms19082392.
Fu R-H, et al. Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 2014;23(4–5):621–30. https://doi.org/10.3727/096368914X678382.
Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29(11):1630–7. https://doi.org/10.1016/j.biomaterials.2007.12.014.
Zhang W, Du A, Liu S, Lv M, Chen S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen Ther. 2021;18:88–96. https://doi.org/10.1016/j.reth.2021.04.002.
Fu Y, et al. Decellularization of porcine skeletal muscle extracellular matrix for the formulation of a matrix hydrogel: a preliminary study. J Cell Mol Med. 2016;20(4):740–9. https://doi.org/10.1111/jcmm.12776.
Fernández-Pérez J, Ahearne M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci Rep. 2019;9(1). https://doi.org/10.1038/s41598-019-49575-2.
Sackett SD, et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci Rep. 2018;8(1):10452. https://doi.org/10.1038/s41598-018-28857-1.
Pouliot RA, et al. Development and characterization of a naturally derived lung extracellular matrix hydrogel. J Biomed Mater Res A. 2016;104(8):1922–35. https://doi.org/10.1002/jbm.a.35726.
Wolf MT, et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33(29):7028–38. https://doi.org/10.1016/j.biomaterials.2012.06.051.
Liguori GR, et al. Abstract 14119: Decellularized arterial extracellular matrix-based hydrogel supports 3D bioprinting of the media layer of small-caliber blood vessels. Circulation. 2019;140(Suppl_1):A14119–A14119. https://doi.org/10.1161/circ.140.suppl_1.14119.
Stern MM, et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30(12):2393–9. https://doi.org/10.1016/j.biomaterials.2008.12.069.
DeQuach JA, et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE. 2010;5(9):e13039. https://doi.org/10.1371/journal.pone.0013039.
Ungerleider JL, Dzieciatkowska M, Hansen KC, Christman KL. Tissue specific muscle extracellular matrix hydrogel improves skeletal muscle regeneration in vivo over non-matched tissue source. 2020. https://doi.org/10.1101/2020.06.30.181164.
Zhang Y, et al. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials. 2009;30(23–24):4021–8. https://doi.org/10.1016/j.biomaterials.2009.04.005.
Choi Y-J, et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials. 2019;206:160–9. https://doi.org/10.1016/j.biomaterials.2019.03.036.
Nguyen MM, Gianneschi NC, Christman KL. Developing injectable nanomaterials to repair the heart. Curr Opin Biotechnol. 2015;34:225–31. https://doi.org/10.1016/j.copbio.2015.03.016.
Hernandez MJ, Christman KL. Designing acellular injectable biomaterial therapeutics for treating myocardial infarction and peripheral artery disease. JACC Basic Transl Sci. 2017;2(2):212–26. https://doi.org/10.1016/j.jacbts.2016.11.008.
Gaetani R, Ungerleider J, Christman KL. Chapter 25 - Acellular injectable biomaterials for treating cardiovascular disease. In Stem Cell and Gene Therapy for Cardiovascular Disease, E. C. Perin, L. W. Miller, D. A. Taylor, and J. T. Willerson, Eds., Boston: Academic Press; 2016. pp. 309–325. https://doi.org/10.1016/B978-0-12-801888-0.00025-4.
DeQuach JA, et al. Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model. Eur Cell Mater. 2012;23:400–412; discussion 412. https://doi.org/10.22203/ecm.v023a31.
Ungerleider JL, et al. Extracellular matrix hydrogel promotes tissue remodeling, arteriogenesis, and perfusion in a rat hindlimb ischemia model. JACC: Basic Transl Sci. 2016;1(1):32–44. https://doi.org/10.1016/j.jacbts.2016.01.009.
Rao N, et al. Engineering an injectable muscle-specific microenvironment for improved cell delivery using a nanofibrous extracellular matrix hydrogel. ACS Nano. 2017;11(4):3851–9. https://doi.org/10.1021/acsnano.7b00093.
Quarta M, et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat Commun. 2017;8:15613. https://doi.org/10.1038/ncomms15613.
Jana S, Levengood SKL, Zhang M. Anisotropic materials for skeletal-muscle-tissue engineering. Adv Mater. 2016;28(48):10588–612. https://doi.org/10.1002/adma.201600240.
Nakayama KH, et al. Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. npj Regen Med. 2018;3(1). https://doi.org/10.1038/s41536-018-0054-3.
Nakayama KH, et al. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun Biol. 2019;2(1). https://doi.org/10.1038/s42003-019-0416-4.
Ostrovidov S, et al. Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng Part B Rev. 2014;20(5):403–36. https://doi.org/10.1089/ten.TEB.2013.0534.
Ostrovidov S, et al. Three dimensional bioprinting in skeletal muscle tissue engineering. Small. 2019;15(24):e1805530. https://doi.org/10.1002/smll.201805530.
Choi Y-J, et al. 3D cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Adv Healthc Mater. 2016;5(20):2636–45. https://doi.org/10.1002/adhm.201600483.
Traverse JH, et al. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl Sci. 2019;4(6):659–69. https://doi.org/10.1016/j.jacbts.2019.07.012.
Urciuolo A, et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci Rep. 2018;8(1):8398. https://doi.org/10.1038/s41598-018-26371-y.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–43. https://doi.org/10.1016/j.biomaterials.2011.01.057.
Bordbar S, LotfiBakhshaiesh N, Khanmohammadi M, Sayahpour FA, Alini M, BaghabanEslaminejad M. Production and evaluation of decellularized extracellular matrix hydrogel for cartilage regeneration derived from knee cartilage. J Biomed Mater Res A. 2020;108(4):938–46. https://doi.org/10.1002/jbm.a.36871.
Wong ML, Griffiths LG. Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization. Acta Biomater. 2014;10(5):1806–16. https://doi.org/10.1016/j.actbio.2014.01.028.
Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263(33):17755–62. https://doi.org/10.1016/S0021-9258(19)77900-9.
Sandrin MS, McKenzie IF. Gal alpha (1,3)Gal, the major xenoantigen(s) recognised in pigs by human natural antibodies. Immunol Rev. 1994;141:169–90. https://doi.org/10.1111/j.1600-065x.1994.tb00877.x.
Joziasse DH, Oriol R. Xenotransplantation: the importance of the Galα1,3Gal epitope in hyperacute vascular rejection. Biochim Biophys Acta (BBA) - Mol Basis Dis. 1999;1455(2):403–418. https://doi.org/10.1016/S0925-4439(99)00056-3.
Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109–16. https://doi.org/10.1016/j.smim.2007.11.003.
Galili U. Acceleration of wound healing by -gal nanoparticles interacting with the natural anti-gal antibody. J Immunol Res. 2015;2015:e589648. https://doi.org/10.1155/2015/589648.
Lu Y, et al. A standardized quantitative method for detecting remnant alpha-Gal antigen in animal tissues or animal tissue-derived biomaterials and its application. Sci Rep. 2018;8(1). https://doi.org/10.1038/s41598-018-32959-1.
Gao H-W, et al. Quantification of α-Gal Antigen Removal in the Porcine Dermal Tissue by α-Galactosidase. Tissue Eng Part C Methods. 2015;21(11):1197–204. https://doi.org/10.1089/ten.TEC.2015.0129.
Wang RM, et al. Humanized mouse model for assessing the human immune response to xenogeneic and allogeneic decellularized biomaterials. Biomaterials. 2017;129:98–110. https://doi.org/10.1016/j.biomaterials.2017.03.016.
Stahl E, et al. Evaluation of the host immune response to decellularized lung scaffolds derived from α-Gal knockout pigs in a non-human primate model. Biomaterials. 2018;87. https://doi.org/10.1016/j.biomaterials.2018.09.038.
Tao M, et al. Sterilization and disinfection methods for decellularized matrix materials: Review, consideration and proposal. Bioact Mater. 2021;6(9):2927–45. https://doi.org/10.1016/j.bioactmat.2021.02.010.
Smoak MM, Mikos AG. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Materials Today Bio. 2020;7:100069.
Anthony A, Robert L, Tony M, Robert N. Principles of Regenerative Medicine. 3rd Edition. 2019. Elsevier. https://doi.org/10.1016/C2015-0-02433-9
Zhuang P, An J, Chua CK, Tan LP. Bioprinting of 3D in vitro skeletal muscle models: A review. Mater Des. 2020;193:108794.
Lu Y, et al. A standardized quantitative method for detecting remnant alpha-Gal antigen in animal tissues or animal tissue-derived biomaterials and its application. Sci Rep. 2018;8:15424.
Philips C, Terrie L, Thorrez L. Decellularized skeletal muscle: a versatile biomaterial in tissue engineering and regenerative medicine. Biomaterials. 2022;283:121436.
Funding
This work was supported by funding from NIH T32 AR079114/AR/NIAMS and 1332329/EFRI/NSF. Mohammed A. Barajaa was funded by Imam Abdulrahman Bin Faisal University, Dammam, 34212, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
The manuscript was conceptualized and written through the contributions of all authors. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barajaa, M.A., Ghosh, D. & Laurencin, C.T. Decellularized Extracellular Matrix-Derived Hydrogels: a Powerful Class of Biomaterials for Skeletal Muscle Regenerative Engineering Applications. Regen. Eng. Transl. Med. (2023). https://doi.org/10.1007/s40883-023-00328-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40883-023-00328-8