Abstract
Purpose
Osteoarthritis (OA) is a global musculoskeletal disorder that affects primarily the knee and hip joints without any FDA-approved disease-modifying therapies. Animal models are essential research tools in developing therapies for OA; many animal studies have provided data for the initiation of human clinical trials. Despite this, there is still a need for strategies to recapitulate the human experience using animal models to better develop treatments and understand pathogenesis. Since our last review on animal models of osteoarthritis in 2016, there have been exciting updates in OA research and models. The main purpose of this review is to update the latest animal models and key features of studies in OA research.
Method
We used our existing classification method and screened articles in PubMed and bibliographic search for animal OA models between 2016 and 2023. Relevant and high-cited articles were chosen for inclusion in this narrative review.
Results
Recent studies were analyzed and classified. We also identified ex vivo models as an area of ongoing research. Each animal model offers its own benefit in the study of OA and there are a full range of outcome measures that can be assessed. Despite the vast number of models, each has its drawbacks that have limited translating approved therapies for human use.
Conclusion
Depending on the outcome measures and objective of the study, researchers should pick the best model for their work. There have been several exciting studies since 2016 that have taken advantage of regenerative engineering techniques to develop therapies and better understand OA.
Lay Summary
Osteoarthritis (OA) is a chronic debilitating disease without any cure that affects mostly the knee and hip joints and often results in surgical joint replacement. Cartilage protects the joint from mechanical forces and degrades with age or in response to injury. The many contributing causes of OA are still being investigated, and animals are used for preclinical research and to test potential new treatments. A single consensus OA animal model for preclinical studies is non-existent. In this article, we review the many animal models for OA and provide a much-needed update on studies and model development since 2016.


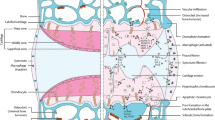
Adapted from Esdaille et al. [25]

Adapted from Ringe et al. [50]


Similar content being viewed by others
References
Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. Arthritis Rheumatol. 2022;74(7):1172–83.
Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation — United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2017;66(9):246–53.
Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105(1):185–99.
Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–8.
Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly, the Framingham osteoarthritis study. Arthritis Rheum. 1995;38(10):1500–5.
Roman-Blas JA, Castañeda S, Largo R, Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther. 2009;11(5):241.
Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9(1):132.
Evangelou E, Chapman K, Meulenbelt I, Karassa FB, Loughlin J, Carr A, et al. Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum. 2009;60(6):1710–21.
Gao B, Cordova ML, (Nigel) Zheng N. Three-dimensional joint kinematics of ACL-deficient and ACL-reconstructed knees during stair ascent and descent. Human Mov Sci. 2012;31(1):222–35.
Croft P, Coggon D, Cruddas M, Cooper C. Osteoarthritis of the hip: an occupational disease in farmers. BMJ. 1992;304(6837):1269–72.
Rodriguez M, Garcia E, Dickens J. Primary and posttraumatic knee osteoarthritis in the military. J Knee Surg. 2019;32(02):134–7.
Farrokhi S, Mazzone B, Yoder A, Grant K, Wyatt M. A narrative review of the prevalence and risk factors associated with development of knee osteoarthritis after traumatic unilateral lower limb amputation. Mil Med. 2016;181(S4):38–44.
Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KDJ. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57–63.
Dantas LO, de Fatima Salvini T, McAlindon TE. Knee osteoarthritis: key treatments and implications for physical therapy. Braz J Phys Ther. 2021;25(2):135–46.
Yaftali NA, Weber K. Corticosteroids and hyaluronic acid injections. Clin Sports Med. 2019;38(1):1–15.
Oo WM, Little C, Duong V, Hunter DJ. The development of disease-modifying therapies for osteoarthritis (DMOADs): the evidence to date. DDDT. 2021;15:2921–45.
Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. The Lancet. 2015;386(9991):376–87.
Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311.
Jiang Y. Osteoarthritis year in review 2021: biology. Osteoarthr Cartil. 2022;30(2):207–15.
Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis — an untreatable disease? Nat Rev Drug Discov. 2005;4(4):331–44.
Donell S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019;4(6):221–9.
King LK, March L, Anandacoomarasamy A. Obesity & osteoarthritis. Indian J Med Res. 2013;138(2):185–93.
Cope PJ, Ourradi K, Li Y, Sharif M. Models of osteoarthritis: the good, the bad and the promising. Osteoarthr Cartil. 2019;27(2):230–9.
Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11(1):19.
Esdaille CJ, Ude CC, Laurencin CT. Regenerative engineering animal models for knee osteoarthritis. Regen Eng Transl Med. 2022;8(2):284–97.
Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19(3):142–6.
Mastbergen SC, Pollmeier M, Fischer L, Vianen ME, Lafeber FPJG. The groove model of osteoarthritis applied to the ovine fetlock joint. Osteoarthr Cartil. 2008;16(8):919–28.
Bertone AL. 15 - Distal limb: Fetlock and pastern. In: Hinchcliff KW, Kaneps AJ, Geor RJ, editors. Equine sports medicine and surgery (2nd edition) [Internet]. W.B. Saunders; 2014. pp. 275–96. Available from: https://www.sciencedirect.com/science/article/pii/B9780702047718000156.
Nicola TL, Jewison DJ. The anatomy and biomechanics of running. Clin Sports Med. 2012;31(2):187–201.
Malda J, Benders KEM, Klein TJ, de Grauw JC, Kik MJL, Hutmacher DW, et al. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr Cartil. 2012;20(10):1147–51.
Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthr Cartil. 2009;17(6):705–13.
Dias IR, Viegas CA, Carvalho PP. Large animal models for osteochondral regeneration. Adv Exp Med Biol. 2018;1059:441–501.
Bertone AL, Ishihara A, Zekas LJ, Wellman ML, Lewis KB, Schwarze RA, et al. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am J Vet Res. 2014;75(2):141–51.
Shakouri SK, Dolati S, Santhakumar J, Thakor AS, Yarani R. Autologous conditioned serum for degenerative diseases and prospects. Growth Factors. 2021;39(1–6):59–70.
Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1):105–15.
McCoy AM. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol. 2015;52(5):803–18.
Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532–53.
Zhou J, Wang Y, Liu Y, Zeng H, Xu H, Lian F. Adipose derived mesenchymal stem cells alleviated osteoarthritis and chondrocyte apoptosis through autophagy inducing. J Cell Biochem. 2019;120(2):2198–212.
Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–30.
Miller RE, Malfait AM. Osteoarthritis pain: what are we learning from animal models? Best Pract Res Clin Rheumatol. 2017;31(5):676–87.
Zaki S, Blaker CL, Little CB. OA foundations – experimental models of osteoarthritis. Osteoarthr Cartil. 2022;30(3):357–80.
Macfadyen MA, Daniel Z, Kelly S, Parr T, Brameld JM, Murton AJ, et al. The commercial pig as a model of spontaneously-occurring osteoarthritis. BMC Musculoskelet Disord. 2019;20(1):70.
Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthr Cartil. 2010;18:S35-52.
Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil. 2010;18(Suppl 3):S17-23.
Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr Cartil. 2010;18(Suppl 3):S24-34.
Laverty S, Girard CA, Williams JM, Hunziker EB, Pritzker KPH. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthr Cartil. 2010;18(Suppl 3):S53-65.
McIlwraith CW, Frisbie DD, Kawcak CE, Fuller CJ, Hurtig M, Cruz A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr Cartil. 2010;18(Suppl 3):S93-105.
Little CB, Smith MM, Cake MA, Read RA, Murphy MJ, Barry FP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthr Cartil. 2010;18(Suppl 3):S80-92.
Cook JL, Kuroki K, Visco D, Pelletier JP, Schulz L, Lafeber FPJG. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr Cartil. 2010;18(Suppl 3):S66-79.
Ringe J, Hemmati-Sadeghi S, Fröhlich K, Engels A, Reiter K, Dehne T, et al. CCL25-supplemented hyaluronic acid attenuates cartilage degeneration in a guinea pig model of knee osteoarthritis. J Orthop Res. 2019;37(8):1723–9.
Wallace IJ, Bendele AM, Riew G, Frank EH, Hung HH, Holowka NB, et al. Physical inactivity and knee osteoarthritis in guinea pigs. Osteoarthr Cartil. 2019;27(11):1721–8.
Miller RE, Lu Y, Tortorella MD, Malfait AM. Genetically engineered mouse models reveal the importance of proteases as osteoarthritis drug targets. Curr Rheumatol Rep. 2013;15(8):350.
Burt PM, Xiao L, Doetschman T, Hurley MM. Ablation of low-molecular-weight FGF2 isoform accelerates murine osteoarthritis while loss of high-molecular-weight FGF2 isoforms offers protection. J Cell Physiol. 2019;234(4):4418–31.
Evans CH, Ghivizzani SC, Robbins PD. Gene delivery to joints by intra-articular injection. Hum Gene Ther. 2018;29(1):2–14.
Little CB, Hunter DJ. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat Rev Rheumatol. 2013;9(8):485–97.
Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25(5):578–92.
Chang JC, Christiansen BA, Murugesh DK, Sebastian A, Hum NR, Collette NM, et al. SOST/Sclerostin improves posttraumatic osteoarthritis and inhibits MMP2/3 expression after injury. J Bone Miner Res. 2018;33(6):1105–13.
Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JHN, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2012;20(7):773–82.
Ko FC, Dragomir CL, Plumb DA, Hsia AW, Adebayo OO, Goldring SR, et al. Progressive cell-mediated changes in articular cartilage and bone in mice are initiated by a single session of controlled cyclic compressive loading. J Orthop Res. 2016;34(11):1941–9.
de Oloveira Martins LP, dos Santos FF, Costa TED, Lacerda ACR, dos Santos JM, Costa KB, et al. Photobiomodulation therapy (light-emitting diode 630 nm) favored the oxidative stress and the preservation of articular cartilage in an induced knee osteoarthritis model. Photobiomodulation, Photomed Laser Surg. 2021;39(4):272–9.
Pitcher T, Sousa-Valente J, Malcangio M. The monoiodoacetate model of osteoarthritis pain in the mouse. JoVE. 2016;111:53746.
Kobayashi K, Imaizumi R, Sumichika H, Tanaka H, Goda M, Fukunari A, et al. Sodium Iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci. 2003;65(11):1195–9.
Miyamoto S, Nakamura J, Ohtori S, Orita S, Omae T, Nakajima T, et al. Intra-articular injection of mono-iodoacetate induces osteoarthritis of the hip in rats. BMC Musculoskelet Disord. 2016;17(1):132.
Zhang RX, Ren K, Dubner R. Osteoarthritis pain mechanisms: basic studies in animal models. Osteoarthr Cartil. 2013;21(9):1308–15.
Micheli L, Di Cesare ML, Lucarini E, Cialdai F, Vignali L, Ghelardini C, et al. Photobiomodulation therapy by NIR laser in persistent pain: an analytical study in the rat. Lasers Med Sci. 2017;32(8):1835–46.
Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158(12):2442–51.
Ikeuchi M, Izumi M, Aso K, Sugimura N, Kato T, Tani T. Effects of intra-articular hyaluronic acid injection on immunohistochemical characterization of joint afferents in a rat model of knee osteoarthritis. EJP. 2015;19(3):334–40.
Çağlar C, Kara H, Ateş O, Uğurlu M. Evaluation of different intraarticular injection therapies with gait analysis in a rat osteoarthritis model. Cartilage. 2021;13(2_suppl):1134S-1143S.
More AS, Kumari RR, Gupta G, Lingaraju MC, Balaganur V, Pathak NN, et al. Effect of iNOS inhibitor S-methylisothiourea in monosodium iodoacetate-induced osteoathritic pain: implication for osteoarthritis therapy. Pharmacol Biochem Behav. 2013;103(4):764–72.
Kim Y, Kim EH, Lee KS, Lee K, Park SH, Na SH, et al. The effects of intra-articular resiniferatoxin on monosodium iodoacetate-induced osteoarthritic pain in rats. Korean J Physiol Pharmacol. 2016;20(1):129–36.
Shah S, Esdaille CJ, Bhattacharjee M, Kan HM, Laurencin CT. The synthetic artificial stem cell (SASC):shifting the paradigm of cell therapy in regenerative engineering. Proc Natl Acad Sci USA. 2022;119(2):e2116865118
Bhattacharjee M, Escobar Ivirico JL, Kan HM, Shah S, Otsuka T, Bordett R, et al. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc Natl Acad Sci USA. 2022;119(4):e2120968119
Khanal M, Gohil SV, Kuyinu E, Kan HM, Knight BE, Baumbauer KM, et al. Injectable nanocomposite analgesic delivery system for musculoskeletal pain management. Acta Biomater. 2018;1(74):280–90.
Britti D, Crupi R, Impellizzeri D, Gugliandolo E, Fusco R, Schievano C, et al. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet Res. 2017;13(1):229.
Malek N, Borowczyk J, Kostrzewa M, Pawlowska A, Drukala J, Starowicz K. The impact of JWH-133 on articular cartilage regeneration in osteoarthritis via metalloproteinase 13-dependent mechanism. Cannabis and Cannabinoid Research. 2022. https://doi.org/10.1089/can.2022.0107
Di Paola R, Fusco R, Impellizzeri D, Cordaro M, Britti D, Morittu VM, et al. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res Ther. 2016;18(1):291.
Kim SE, Lee JY, Shim KS, Lee S, Min K, Bae JH, et al. Attenuation of inflammation and cartilage degradation by sulfasalazine-containing hyaluronic acid on osteoarthritis rat model. Int J Biol Macromol. 2018;114:341–8.
Chen L, Lou Y, Pan Z, Cao X, Zhang L, Zhu C, et al. Treadmill and wheel exercise protect against JNK/NF-κB induced inflammation in experimental models of knee osteoarthritis. Biochem Biophys Res Commun. 2020;523(1):117–22.
Allen J, Imbert I, Havelin J, Henderson T, Stevenson G, Liaw L, et al. Effects of treadmill exercise on advanced osteoarthritis pain in rats: exercise reverses osteoarthritis pain and bone remodeling. Arthritis Rheumatol. 2017;69(7):1407–17.
Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011;12(1):134.
Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004;370(2–3):236–40.
van Osch GJVM, Blankevoort L, van der Kraan PM, Janssen B, Hekman E, Huiskes R, et al. Laxity characteristics of normal and pathological murine knee jointsin vitro. J Orthop Res. 1995;13(5):783–91.
Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthr Cartil. 1998;6(3):177–86.
Lubis AMT, Wonggokusuma E, Marsetio AF. Intra-articular recombinant human growth hormone injection compared with hyaluronic acid and placebo for an osteoarthritis model of New Zealand rabbits. Knee Surg Relat Res. 2019;31(1):44–53.
Park J, Lee J, Kim KI, Lee J, Jang S, Choi HT, et al. A pathophysiological validation of collagenase II-induced biochemical osteoarthritis animal model in rabbit. Tissue Eng Regen Med. 2018;15(4):437–44.
Farr J, Gomoll AH, Yanke AB, Strauss EJ, Mowry KC, ASA Study Group. A randomized controlled single-blind study demonstrating superiority of amniotic suspension allograft injection over hyaluronic acid and saline control for modification of knee osteoarthritis symptoms. J Knee Surg. 2019;32(11):1143–54.
Gomoll AH, Farr J, Cole BJ, Flanigan DC, Lattermann C, Mandelbaum BR, et al. Safety and efficacy of an amniotic suspension allograft injection over 12 months in a single-blinded, randomized controlled trial for symptomatic osteoarthritis of the knee. Arthroscopy: J Arthrosc Relat Surg. 2021;37(7):2246–57.
Collins KH, Lenz KL, Pollitt EN, Ferguson D, Hutson I, Springer LE, et al. Adipose tissue is a critical regulator of osteoarthritis. Proc Natl Acad Sci U S A. 2021;118(1):e2021096118.
Griffin TM, Batushansky A, Hudson J, Lopes EBP. Correlation network analysis shows divergent effects of a long-term, high-fat diet and exercise on early stage osteoarthritis phenotypes in mice. J Sport Health Sci. 2020;9(2):119–31.
Hahn AK, Batushansky A, Rawle RA, Prado Lopes EB, June RK, Griffin TM. Effects of long-term exercise and a high-fat diet on synovial fluid metabolomics and joint structural phenotypes in mice: an integrated network analysis. Osteoarthr Cartil. 2021;29(11):1549–63.
Lorenz W, Buhrmann C, Mobasheri A, Lueders C, Shakibaei M. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: implications for the development of rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R111.
Yoshino S, Sasatomi E, Ohsawa M. Bacterial lipopolysaccharide acts as an adjuvant to induce autoimmune arthritis in mice. Immunology. 2000;99(4):607–14.
Huang Z, Kraus VB. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol. 2016;12(2):123–9.
Neuenschwander HM, Moreira JJ, Vendruscolo CP, Fülber J, Seidel SRT, Michelacci YM, et al. Hyaluronic acid has chondroprotective and joint-preserving effects on LPS-induced synovitis in horses. J Vet Sci. 2019;20(6):e67.
Molinet M, Alves N, Vasconcelos A, Deana NF. Comparative study of osteoarthritis induced by monoiodoacetate and papain in rabbit temporomandibular joints: macroscopic and microscopic analysis. Folia Morphol (Warsz). 2020;79(3):516–27.
Cheng F, Yan FF, Liu YP, Cong Y, Sun KF, He XM. Dexmedetomidine inhibits the NF-κB pathway and NLRP3 inflammasome to attenuate papain-induced osteoarthritis in rats. Pharm Biol. 2019;57(1):649–59.
Lindström E, Rizoska B, Tunblad K, Edenius C, Bendele AM, Maul D, et al. The selective cathepsin K inhibitor MIV-711 attenuates joint pathology in experimental animal models of osteoarthritis. J Transl Med. 2018;16(1):56.
Jin Y, Koh RH, Kim SH, Kim KM, Park GK, Hwang NS. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater Sci Eng, C. 2020;115:111096.
Lee MI, Kim JH, Kwak HH, Woo HM, Han JH, Yayon A, et al. A placebo-controlled study comparing the efficacy of intra-articular injections of hyaluronic acid and a novel hyaluronic acid-platelet-rich plasma conjugate in a canine model of osteoarthritis. J Orthop Surg Res. 2019;14(1):314.
Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–81.
Veronesi F, Vandenbulcke F, Ashmore K, Di Matteo B, Nicoli Aldini N, Martini L, et al. Meniscectomy-induced osteoarthritis in the sheep model for the investigation of therapeutic strategies: a systematic review. Int Orthop. 2020;44(4):779–93.
Tawonsawatruk T, Sriwatananukulkit O, Himakhun W, Hemstapat W. Comparison of pain behaviour and osteoarthritis progression between anterior cruciate ligament transection and osteochondral injury in rat models. Bone Joint Res. 2018;7(3):244–51.
Wang Y, Chen Y, Wei Y. Osteoarthritis animal models for biomaterial-assisted osteochondral regeneration. Biomater Transl. 2022;3(4):264–79.
Liu Y, Dzidotor G, Le TT, Vinikoor T, Morgan K, Curry EJ, et al. Exercise-induced piezoelectric stimulation for cartilage regeneration in rabbits. Sci Transl Med. 2022;14(627):eabi7282.
Park S, Kang S, Kim DS, Zhang T. Protection against osteoarthritis symptoms by aerobic exercise with a high-protein diet by reducing inflammation in a testosterone-deficient animal model. Life. 2022;12(2):177.
Atallah A, Mhaouty-Kodja S, Grange-Messent V. Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice. J Cereb Blood Flow Metab. 2017;37(9):3161–75.
Kloner RA, Carson C, Dobs A, Kopecky S, Mohler ER. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67(5):545–57.
Li H, Gou Y, Tian F, Zhang Y, Lian Q, Hu Y, et al. Combination of metformin and exercise alleviates osteoarthritis in ovariectomized mice fed a high-fat diet. Bone. 2022;157:116323.
Kim JE, Song DH, Kim SH, Jung Y, Kim SJ. Development and characterization of various osteoarthritis models for tissue engineering. PLoS One. 2018;13(3):e0194288.
Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Ajvr. 2007;68(3):290–6.
Frisbie DD, Al-Sobayil F, Billinghurst RC, Kawcak CE, McIlwraith CW. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthr Cartil. 2008;16(10):1196–204.
Bertoni L, Jacquet-Guibon S, Branly T, Desancé M, Legendre F, Melin M, et al. Evaluation of allogeneic bone-marrow-derived and umbilical cord blood-derived mesenchymal stem cells to prevent the development of osteoarthritis in an equine model. IJMS. 2021;22(5):2499.
Rojas-Ortega M, Cruz R, Vega-López MA, Cabrera-González M, Hernández-Hernández JM, Lavalle-Montalvo C, et al. Exercise modulates the expression of IL-1β and IL-10 in the articular cartilage of normal and osteoarthritis-induced rats. Pathol – Res Pract. 2015;211(6):435–43.
Siebelt M, Waarsing JH, Kops N, Piscaer TM, Verhaar JAN, Oei EHG, et al. Quantifying osteoarthritic cartilage changes accurately using in vivo microCT arthrography in three etiologically distinct rat models: quantifying osteoarthritic cartilage changes. J Orthop Res. 2011;29(11):1788–94.
Li K, Zhang P, Zhu Y, Alini M, Grad S, Li Z. Establishment of an ex vivo inflammatory osteoarthritis model with human osteochondral explants. Front Bioeng Biotechnol. 2021;9:787020.
Kleuskens MWA, van Donkelaar CC, Kock LM, Janssen RPA, Ito K. An ex vivo human osteochondral culture model. J Orthop Res. 2021;39(4):871–9.
Genemaras AA, Ennis H, Bradshaw B, Kaplan L, Huang CYC. Effects of anti-inflammatory agents on expression of early responsive inflammatory and catabolic genes in ex vivo porcine model of acute knee cartilage injury. Cartilage. 2018;9(3):293–303.
Lai-Zhao Y, Pitchers KK, Appleton CT. Transient anabolic effects of synovium in early post-traumatic osteoarthritis: a novel ex vivo joint tissue co-culture system for investigating synovium-chondrocyte interactions. Osteoarthr Cartil. 2021;29(7):1060–70.
Mouser VHM, Dautzenberg NMM, Levato R, van Rijen MHP, Dhert WJA, Malda J, et al. Ex vivo model unravelling cell distribution effect in hydrogels for cartilage repair. Altex. 2018;35(1):65–76.
O’Brien M, Philpott HT, McDougall JJ. Understanding osteoarthritis pain through animal models. Clin Exp Rheumatol. 2017;35 Suppl 107(5):47–52.
Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–93.
Li Q, Amano K, Link TM, Ma CB. Advanced imaging in osteoarthritis. Sports Health. 2016;8(5):418–28.
Burge AJ, Jawetz ST. Advanced magnetic resonance imaging in osteoarthritis. Semin Musculoskelet Radiol. 2020;24(4):355–66.
Oğuz R, Belviranlı M, Okudan N. Effects of exercise training alone and in combination with kinesio taping on pain, functionality, and biomarkers related to the cartilage metabolism in knee osteoarthritis. Cartilage. 2021;13(1_suppl):1791S-1800S.
Hu X, Ni S, Zhao K, Qian J, Duan Y. Bioinformatics-led discovery of osteoarthritis biomarkers and inflammatory infiltrates. Front Immunol. 2022;13:871008.
McIlwraith CW, Kawcak CE, Frisbie DD, Little CB, Clegg PD, Peffers MJ, et al. Biomarkers for equine joint injury and osteoarthritis. J Orthop Res. 2018;36(3):823–31.
Li P, Che X, Gao Y, Zhang R. Proteomics and bioinformatics analysis of cartilage in post-traumatic osteoarthritis in a mini-pig model of anterior cruciate ligament repair. Med Sci Monit. 2020;9(26):e920104.
Grote CW, Mackay MJ, Lu Q, Liu X, Meyer AR, Wang J. A whole-joint histopathologic grading system for murine knee osteoarthritis. J Orthop Res. 2023;41(7):1407–18. https://doi.org/10.1002/jor.25482.
Li J, Zhang B, Liu WX, Lu K, Pan H, Wang T, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis. 2020;79(5):635–45.
Korchi AM, Cengarle-Samak A, Okuno Y, Martel-Pelletier J, Pelletier JP, Boesen M, et al. Inflammation and hypervascularization in a large animal model of knee osteoarthritis: imaging with pathohistologic correlation. J Vasc Interv Radiol. 2019;30(7):1116–27.
Miller RE, Kim YS, Tran PB, Ishihara S, Dong X, Miller RJ, et al. Visualization of peripheral neuron sensitization in a surgical mouse model of osteoarthritis by in vivo calcium imaging. Arthritis Rheumatol. 2018;70(1):88–97.
Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271(21):12511–6.
Lindström E, Rizoska B, Henderson I, Terelius Y, Jerling M, Edenius C, et al. Nonclinical and clinical pharmacological characterization of the potent and selective cathepsin K inhibitor MIV-711. J Transl Med. 2018;16(1):125.
Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–75.
Legrand CB, Lambert CJ, Comblain FV, Sanchez C, Henrotin YE. Review of soluble biomarkers of osteoarthritis: lessons from animal models. Cartilage. 2017;8(3):211–33.
Li Z, Huang Z, Zhang H, Lu J, Tian Y, Wei Y, et al. P2X7 receptor induces pyroptotic inflammation and cartilage degradation in osteoarthritis via NF-κB/NLRP3 crosstalk. Oxid Med Cell Longev. 2021;2021:8868361.
Cho Y, Jeong S, Kim H, Kang D, Lee J, Kang SB, et al. Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp Mol Med. 2021;53(11):1689–96.
Oo WM, Yu SPC, Daniel MS, Hunter DJ. Disease-modifying drugs in osteoarthritis: current understanding and future therapeutics. Expert Opin Emerg Drugs. 2018;23(4):331–47.
Oo WM. Prospects of disease-modifying osteoarthritis drugs. Clin Geriatr Med. 2022;38(2):397–432.
Malfait AM, Little CB. On the predictive utility of animal models of osteoarthritis. Arthritis Res Ther. 2015;17(1):225.
Salgado C, Jordan O, Allémann E. Osteoarthritis in vitro models: applications and implications in development of intra-articular drug delivery systems. Pharmaceutics. 2021;13(1):60.
Bartolotti I, Roseti L, Petretta M, Grigolo B, Desando G. A roadmap of in vitro models in osteoarthritis: a focus on their biological relevance in regenerative medicine. J Clin Med. 2021;10(9):1920.
Johnson CI, Argyle DJ, Clements DN. In vitro models for the study of osteoarthritis. Vet J. 2016;209:40–9.
Ude CC, Shamsul BS, Ng MH, Chen HC, Ohnmar H, Amaramalar SN, et al. Long-term evaluation of osteoarthritis sheep knee, treated with TGF-β3 and BMP-6 induced multipotent stem cells. Exp Gerontol. 2018;104:43–51.
Drevet S, Favier B, Brun E, Gavazzi G, Lardy B. Mouse models of osteoarthritis: a summary of models and outcomes assessment. Comp Med. 2022;72(1):3–13.
Roemer FW, Jarraya M, Felson DT, Hayashi D, Crema MD, Loeuille D, et al. Magnetic resonance imaging of Hoffa’s fat pad and relevance for osteoarthritis research: a narrative review. Osteoarthr Cartil. 2016;24(3):383–97.
Roemer FW, Guermazi A, Demehri S, Wirth W, Kijowski R. Imaging in osteoarthritis. Osteoarthr Cartil. 2022;30(7):913–34.
Hellio le Graverand MP, Clemmer RS, Redifer P, Brunell RM, Hayes CW, Brandt KD, et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis. 2013;72(2):187–95.
Raeissadat SA, Ghazi Hosseini P, Bahrami MH, Salman Roghani R, Fathi M, Gharooee Ahangar A, et al. The comparison effects of intra-articular injection of platelet rich plasma (PRP), plasma rich in growth factor (PRGF), hyaluronic acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet Disord. 2021;22(1):134.
Bihlet AR, Byrjalsen I, Andersen JR, Öberg F, Herder C, Bowes MA, et al. Symptomatic and structural benefit of cathepsin K inhibition by MIV-711 in a subgroup with unilateral pain: post-hoc analysis of a randomised phase 2a clinical trial. Clin Exp Rheumatol. 2022;40(5):1034–7.
Rocho FR, Bonatto V, Lameiro RF, Lameira J, Leitão A, Montanari CA. A patent review on cathepsin K inhibitors to treat osteoporosis (2011–2021). Expert Opin Ther Pat. 2022;32(5):561–73.
Runger TM, Adami S, Benhamou CL, Czerwiski E, Farrerons J, Kendler DL, et al. Morphea-like skin reactions in patients treated with the cathepsin K inhibitor balicatib. J Am Acad Dermatol. 2012;66(3):e89-96.
Abhishek A, Doherty M. Mechanisms of the placebo response in pain in osteoarthritis. Osteoarthr Cartil. 2013;21(9):1229–35.
Watson A, Power A, Brown C, El-Deredy W, Jones A. Placebo analgesia: cognitive influences on therapeutic outcome. Arthritis Res Ther. 2012;14(2):206.
Kim JS, Borges S, Clauw DJ, Conaghan PG, Felson DT, Fleming TR, et al. FDA/Arthritis Foundation osteoarthritis drug development workshop recap: assessment of long-term benefit. Semin Arthritis Rheum. 2022;56:152070.
Borden M, Attawia M, Khan Y, El-Amin SF, Laurencin CT. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J Bone Joint Surg Br. 2004;86(8):1200–8.
Yu X, Botchwey EA, Levine EM, Pollack SR, Laurencin CT. Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Proc Natl Acad Sci U S A. 2004;101(31):11203–8.
Cushnie EK, Ulery BD, Nelson SJ, Deng M, Sethuraman S, Doty SB, et al. Simple signaling molecules for inductive bone regenerative engineering. PLoS One. 2014;9(7):e101627.
Ogueri KS, Escobar Ivirico JL, Li Z, Blumenfield RH, Allcock HR, Laurencin CT. Synthesis, physicochemical analysis, and side group optimization of degradable dipeptide-based polyphosphazenes as potential regenerative biomaterials. ACS Appl Polym Mater. 2019;1(6):1568–78.
Ambrosio AM, Sahota JS, Khan Y, Laurencin CT. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J Biomed Mater Res. 2001;58(3):295–301.
Arnold AM, Holt BD, Daneshmandi L, Laurencin CT, Sydlik SA. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc Natl Acad Sci U S A. 2019;116(11):4855–60.
Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–21.
Fu R, Bertrand D, Wang J, Kavaseri K, Feng Y, Du T, et al. In vivo and in silico monitoring bone regeneration during distraction osteogenesis of the mouse femur. Comput Methods Programs Biomed. 2022;216:106679.
Mao L, Wu W, Wang M, Guo J, Li H, Zhang S, et al. Targeted treatment for osteoarthritis: drugs and delivery system. Drug Deliv. 2021;28(1):1861–76.
Kang LJ, Yoon J, Rho JG, Han HS, Lee S, Oh YS, et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275:120967.
Ma Y, Yang H, Zong X, Wu J, Ji X, Liu W, et al. Artificial M2 macrophages for disease-modifying osteoarthritis therapeutics. Biomaterials. 2021;274:120865.
Abdel-Aziz MA, Ahmed HMS, El-Nekeety AA, Sharaf HA, Abdel-Aziem SH, Abdel-Wahhab MA. Biosynthesis of gold nanoparticles for the treatment of osteoarthritis alone or in combination with Diacerein(®) in a rat model. Inflammopharmacology. 2021;29(3):705–19.
Saveh Shemshaki N, Kan HM, Barajaa M, Otsuka T, Lebaschi A, Mishra N, et al. Muscle degeneration in chronic massive rotator cuff tears of the shoulder:addressing the real problem using a graphene matrix. Proc Natl Acad Sci U S A. 2022;119(33):e2208106119.
Cooper JAJ, Sahota JS, Gorum WJ 2nd, Carter J, Doty SB, Laurencin CT. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci U S A. 2007;104(9):3049–54.
Mengsteab PY, Otsuka T, McClinton A, Shemshaki NS, Shah S, Kan HM, et al. Mechanically superior matrices promote osteointegration and regeneration of anterior cruciate ligament tissue in rabbits. Proc Natl Acad Sci U S A. 2020;117(46):28655–66.
Acknowledgements
We would like to thank our funding source NIH/NIAMS T32AR079114 (To CTL) and Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chapman, J.H., Ghosh, D., Attari, S. et al. Animal Models of Osteoarthritis: Updated Models and Outcome Measures 2016–2023. Regen. Eng. Transl. Med. (2023). https://doi.org/10.1007/s40883-023-00309-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40883-023-00309-x