Abstract
Purpose
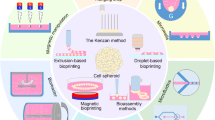
One of the major challenges in cell-laden microgel bioprocessing is to design an effective method of cell encapsulation in the biomaterial carrier while retaining high cell viability and ensuring small enough particles for injectability. In this study we aim to compare four bioprocessing techniques for making hydrogel microcarriers, including by emulsification gelation and dropwise gelation approaches.
Methods
A Pluronic-Fibrinogen (FF-127) hydrogel biomaterial was used to make the microgels based on a lower critical solubility temperature (LSCT) phase transition. Additional cross-linking of the hydrogels was achieved using light-activated photochemistry (i.e., photopolymerization). The four bioprocessing methodologies include emulsification gelation in oil (with and without dual photo-initiator free-radical polymerization), reverse thermal gelation (in warm cell culture media), dropwise gelation through a vibrating needle device, and dropwise gelation through an atomization device (in warm cell culture media gelation baths). The microgels made with each method were characterized with and without cells; comparisons of microgel size and cell growth were reported.
Results
The dual photo-initiator emulsification technique produced FF-127 spherical microgels with an average diameter of 222 and 256 μm, with and without cells, respectively. The reverse thermal encapsulation produced irregularly shaped microgels with an average diameter of 241 and 702 μm, with and without cells, respectively. The vibrating needle and atomization techniques produced irregularly shaped microgels with an average diameter of 195 and 151 μm without cells, respectively, and 464 and 332 μm with cells, respectively. The viability of fibroblasts in the microgels was high after 24 h, except for those treatments that underwent photo-polymerization (i.e., emulsification photo-polymerization and vibrating needle with photo-polymerization). The cells remained viable for up to 3 weeks in culture and spread three-dimensionally in the microgels over this time course.
Conclusions
The rapid temperature-induced phase transition of the FF-127 enables the formation of microgels either through dropwise gelation or by emulsification, both through physical cross-linking. The use of a free-radical polymerization cross-linking reaction was more cyto-toxic to the cells as compared to the physical cross-linking by reverse thermal gelation alone. The average microgel size in all the techniques was significantly smaller and more uniform when producing the microgels without cells as compared to with cells. The reverse thermal gelation technique produced cell-laden microgels with the least amount of specialized equipment and bioprocessing steps of all the methods reported.
Lay Summary
This study provides the framework for producing cell-laden microgels that are of a sufficiently small diameter to be used for injectable cell therapy. The challenge in this regard is to design a simple, scalable, and efficient methods of cell encapsulation in the biomaterials, retaining high cell viability and ensuring small enough particles for injectability. For this purpose, we evaluated four methods that are commonly applied in microgel bioprocessing, and tested these with two types of cells using hydrogels that exhibit lower critical solubility temperature (LCST) properties. This investigation has enabled us to identify advantages and disadvantages for each system of bioprocessing of cell-laden microgels.







Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Wei X, et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34(6):747–54.
Saadi T, et al. Cellularized biosynthetic microhydrogel polymers for intravascular liver tissue regeneration therapy. Tissue Eng Part A. 2014;20(21–22):2850–9.
Headen DM, et al. Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance. Nat Mater. 2018;17(8):732.
Birman T, Seliktar D. Injectability of biosynthetic hydrogels: consideration for minimally invasive surgical procedures and 3D bioprinting. Adv Funct Mater. 2021;31:29.
Headen DM, et al. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv Mater. 2014;26(19):3003–8.
Burdick JA, Prestwich GD. hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23(12):H41–56.
Coronel MM, et al. Immunotherapy via PD-L1-presenting biomaterials leads to long-term islet graft survival. Sci Adv. 2020;6:35.
Boisserand, LSB., et al. 2016 Biomaterial applications in cell-based therapy in experimental stroke. Stem Cells International, 2016.
Nadlacki B, Suuronen EJ. Biomaterial strategies to improve the efficacy of bone marrow cell therapy for myocardial infarction. Expert Opin Biol Ther. 2016;16(12):1501–16.
Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124–8.
Fuoco, C et al. 3D hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering. Frontiers in Physiology, 2014 5.
Bearzi, C et al. PlGF-MMP9-engineered iPS cells supported on a PEG-fibrinogen hydrogel scaffold possess an enhanced capacity to repair damaged myocardium. Cell Death & Disease, 2014 5.
Rufaihah AJ, et al. Dual delivery of VEGF and ANG-1 in ischemic hearts using an injectable hydrogel. Acta Biomater. 2017;48:58–67.
Lev R, Seliktar D. Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J Royal Soc Inter. 2018;15:138.
Weaver JD, et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci Adv. 2017;3:6.
Yang JA, et al. In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci. 2014;39(12):1973–86.
Daly AC, et al. Hydrogel microparticles for biomedical applications. Nat Rev Mater. 2020;5(1):20–43.
Uman S, Dhand A, Burdick JA. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J Appl Polymer Sci. 2020;137:25.
Guvendiren M, Lu HD, Burdick JA. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8(2):260–72.
Oliveira MB, et al. Injectable PEGylated fibrinogen cell-laden microparticles made with a continuous solvent- and oil-free preparation method. Acta Biomater. 2015;13:78–87.
Chang, S et al. 2020 Emulsion-based encapsulation of pluripotent stem cells in hydrogel microspheres for cardiac differentiation. Biotechnol Prog e2986.
Kerscher, et al. Direct hydrogel encapsulation of pluripotent stem cells enables ontomimetic differentiation and growth of engineered human heart tissues. Biomaterials. 2016;83(383):95.
Seeto WJ, et al. Encapsulation of equine endothelial colony forming cells in highly uniform, injectable hydrogel microspheres for local cell delivery. Tissue Eng Part C Methods. 2017;23(11):815–25.
Weaver JD, et al. Synthetic poly(ethylene glycol)-based microfluidic islet encapsulation reduces graft volume for delivery to highly vascularized and retrievable transplant site. Am J Transplant. 2019;19(5):1315–27.
Franco CL, Price J, West JL. Development and optimization of a dual-photoinitiator, emulsion-based technique for rapid generation of cell-laden hydrogel microspheres. Acta Biomater. 2011;7(9):3267–76.
Goldshmid R, et al. A method for preparation of hydrogel microcapsules for stem cell bioproces sing and stem cell therapy. Methods. 2015;84:35–43.
Goldshmid R, Seliktar D. Hydrogel modulus affects proliferation rate and pluripotency of human mesenchymal stem cells grown in three-dimensional culture. ACS Biomater Sci Eng. 2017;3(12):3433–46.
Mohamed MGA, et al. Microfluidics-based fabrication of cell-laden microgels. Biomicrofluidics. 2020;14:2.
Liu AL, Garcia AJ. Methods for generating hydrogel particles for protein delivery. Ann Biomed Eng. 2016;44(6):1946–58.
Headen, DM., JR. Garcia, AJ. Garcia Parallel droplet microfluidics for high throughput cell encapsulation and synthetic microgel generation. Microsystems & Nanoengineering, 2018 4.
Choe G, et al. Hydrogel biomaterials for stem cell microencapsulation. Polymers. 2018;10:9.
Plotkin M, et al. The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack. Biomaterials. 2014;35(5):1429–38.
Navaro Y, et al. Matrix stiffness determines the fate of nucleus pulposus-derived stem cells. Biomaterials. 2015;49:68–76.
Zhou XC, et al. Human cell encapsulation in gel microbeads with cosynthesized concentric nanoporous solid shells. Adv Funct Mater. 2018;28:21.
Hodgkinson T, et al. Microparticles for controlled growth differentiation factor 6 delivery to direct adipose stem cell-based nucleus pulposus regeneration. J Tissue Eng Regen Med. 2019;13(8):1406–17.
Bratt-Leal AM, et al. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32(1):48–56.
Ivanir E, Shachaf Y, Seliktar D. A model system for screening cancer drugs and evaluating chemosensitivity. J Tissue Eng Regen Med. 2012;6:355–355.
Yosef A, et al. Fibrinogen-based hydrogel modulus and ligand density effects on cell morphogenesis in two-dimensional and three-dimensional cell cultures. Adv Healthcare Mater. 2019;8:13.
Rufaihah, A.J., et al., The effect of scaffold modulus on the morphology and remodeling of fetal mesenchymal stem cells. Frontiers in Physiology, 2018. 9.
Tzouanas SN, et al. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. J Biomed Mater Res, Part A. 2014;102(5):1222–30.
Neffe, A.T., et al., Microparticles from glycidylmethacrylated gelatin as cell carriers prepared in an aqueous two-phase system. European Polymer Journal, 2021. 142.
Pullagura, B.K., S. Amarapalli, and V. Gundabala, Coupling electrohydrodynamics with photopolymerization for microfluidics-based generation of polyethylene glycol diacrylate (PEGDA) microparticles and hydrogels. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2021. 608.
Mironi-Harpaz I, et al. Photopolymerization of cell-encapsulating hydrogels: Crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012;8(5):1838–48.
Zhang XJ, et al. Role of a high calcium ion content in extending the properties of alginate dual-crosslinked hydrogels. J Mater Chem A. 2020;8(47):25390–401.
Karoubi G, et al. Single-cell hydrogel encapsulation for enhanced survival of human marrow stromal cells. Biomaterials. 2009;30(29):5445–55.
Kanda, et al. Deterministic encapsulation of human cardiac stem cells in variable composition nanoporous gel cocoons to enhance therapeutic repair of injured myocardium. Acs Nano. 2018;12(5):4338–50.
Mayfield AE, et al. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35(1):133–42.
Mak WC, et al. Thermo-rheological responsive microcapsules for time-dependent controlled release of human mesenchymal stromal cells. Biomaterials Science. 2017;5(11):2241–50.
Mak WC, et al. Controlled delivery of human cells by temperature responsive microcapsules (vol 6, pg 439, 2015). J Funct Biomater. 2018;9:2.
Cellesi F, et al. Towards a fully synthetic substitute of alginate: Optimization of, a thermal gelation/chemical cross-linking scheme (“Tandem” gelation) for the production of beads and liquid-core capsules. Biotechnol Bioeng. 2004;88(6):740–9.
Cellesi F, Tirelli N, Hubbell JA. Towards a fully-synthetic substitute of alginate: development of a new process using thermal gelation and chemical cross-linking. Biomaterials. 2004;25(21):5115–24.
Shachaf Y, Gonen-Wadmany M, Seliktar D. The biocompatibility of Pluronic (R) F127 fibrinogen-based hydrogels. Biomaterials. 2010;31(10):2836–47.
Cellesi F, Tirelli N, Hubbell JA. Materials for cell encapsulation via a new tandem approach combining reverse thermal gelation and covalent crosslinking. Macromol Chem Phys. 2002;203(10–11):1466–72.
Azagarsamy MA, Anseth KS. Bioorthogonal click chemistry: an indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett. 2013;2(1):5–9.
Liu YS, et al. Fabrication of injectable hydrogels via bio-orthogonal chemistry for tissue engineering. New J Chem. 2020;44(27):11420–32.
Weaver JD, et al. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials. 2018;172:54–65.
Frisman I, et al. Stimulus-Responsive Hydrogels Made From Biosynthetic Fibrinogen Conjugates for Tissue Engineering: Structural Characterization. Langmuir. 2011;27(11):6977–86.
Mironi-Harpaz I, et al. 2015 In-situ architectures designed in 3D cell-laden hydrogels using microscopic laser photolithography. Advanced Mater. 1933;27(11):1933.
Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26(15):2467–77.
Gonen-Wadmany M, Goldshmid R, Seliktar D. Biological and mechanical implications of PEGylating proteins into hydrogel biomaterials. Biomaterials. 2011;32(26):6025–33.
Costantini M, et al. Biofabricating murine and human myo-substitutes for rapid volumetric muscle loss restoration. Embo Molecular Med. 2021;13:3.
Sacchetti B, et al. No identical "mesenchymal stem cells’’ at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports. 2016;6(6):897–913.
Rawle A. The Basic Principles of Particle Size Analysis. Surface Coatings Inter Part A: Coatings J. 2003;86(2):58–65.
Schmidt O, et al. Immobilized fibrinogen in PEG hydrogels does not improve chondrocyte-mediated matrix deposition in response to mechanical stimulation. Biotechnol Bioeng. 2006;95(6):1061–9.
Li RH. Materials for immunoisolated cell transplantation. Adv Drug Deliv Rev. 1998;33(1–2):87–109.
Rabanel JM, et al. Progress technology in microencapsulation methods for cell therapy. Biotechnol Prog. 2009;25(4):946–63.
Steele JAM, et al. Therapeutic cell encapsulation techniques and applications in diabetes. Adv Drug Deliv Rev. 2014;67–68:74–83.
Rang A, et al. Cell encapsulation via microtechnologies. Biomaterials. 2014;35(9):2651–63.
Aguado BA, et al. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18(7–8):806–15.
Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. Journal of Biomaterials Science-Polymer Edition. 2000;11(5):439–57.
Chaemsawang, W., et al., Emulsion cross-linking technique for human fibroblast encapsulation. Inter J Biomater, 2018.
Dressaire E, Sauret A. Clogging of microfluidic systems. Soft Matter. 2017;13(1):37–48.
Chan ES, et al. The effect of low air-to-liquid mass flow rate ratios on the size, size distribution and shape of calcium alginate particles produced using the atomization method. J Food Eng. 2012;108(2):297–303.
Acknowledgements
This research was partially supported by the Israel Science Foundation grant no. 2130/19, the JPND grant 3DPD in association with the Israeli Office of the Chief Scientist, and the Israel Innovation Authority Nofar program. We thank the International Exchange Program 2016–2017 of the University of Rome Tor Vergata and the Association of MAE-MIUR-CRUI Foundation for the Rita Levi Montalcini Award for their financial support for the project.
Funding
This research was partially supported by the Israel Science Foundation grant no. 2130/19, the JPND grant 3DPD in association with the Israeli Chief Scientist Office, and the Israel Innovation Authority Nofar program. Additional financial support was provided from the Association of MAE-MIUR-CRUI Foundation for the Rita Levi Montalcini Award.
Author information
Authors and Affiliations
Contributions
RH and HSY planned and performed experiments, analyzed data, and wrote the manuscript. CG planned experiments related to muscle cell culture. DS planned experiments, analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Skeletal muscle biopsies were obtained from healthy donors following informed consent in line with the guidelines of the WMA Declaration of Helsinki–Ethical Principles For Medical Research Involving Human Subjects.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PPTX 2.672 MB)
Rights and permissions
About this article
Cite this article
Hamami, R., Simaan-Yameen, H., Gargioli, C. et al. Comparison of Four Different Preparation Methods for Making Injectable Microgels for Tissue Engineering and Cell Therapy. Regen. Eng. Transl. Med. 8, 615–629 (2022). https://doi.org/10.1007/s40883-022-00261-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00261-2