Abstract
Frogeye leaf spot (FLS), caused by Cercospora sojina, is an economically important disease of soybean in many parts of the world where soybean is grown, including the United States and Argentina. Yield losses caused by FLS epidemics are mainly due to reduced photosynthetic leaf area, premature defoliation and reduced seed weight. Epidemics may be initiated from inoculum produced on infected plant residue where the pathogen can overwinter for up to two years. Many questions relating to population biology, genetic variability and pathotype structure remain unanswered. Race designations for C. sojina are under debate since the use of the 12 soybean differentials has produced inconsistent numbers of races in separate studies. Although a sexual stage for C. sojina has not been observed in either field or laboratory conditions, equal proportion of mating-type loci and high genotypic diversity suggest that populations of C. sojina are most likely to be undergoing cryptic sexual reproduction. Management practices for this disease include the use of cultivars with partial or complete resistance (Rcs genes), cultural practices (crop rotation and tillage) and foliar fungicide applications. However, resistance to quinone outside inhibitor (QoI) fungicides (caused by the G143A mutation in the Cytochrome b gene) has been reported since 2010, and resistant populations have become widespread across more than 20 soybean-producing states in the U.S. This review provides detailed information on the taxonomy, identification and genetic diversity of the pathogen. It also summarizes epidemiological aspects and strategies to combat this increasing threat to soybean crops worldwide.
Similar content being viewed by others
Introduction
Cercospora sojina Hara is the causal agent of frogeye leaf spot (FLS) on soybean (Glycine max) (Athow and Probst 1952). The disease was first reported in Japan in 1915, in the United States in 1924, in Brazil in 1971, and in Argentina in 1983 (Melchers 1925; Lehman 1928; Veiga and Kimati 1974; Giorda and Justh 1983). In the United States, the disease historically has been most common in the southern soybean production region, and recently has become more common in the midwestern and northern soybean production regions of the country, including Iowa, Nebraska, North Dakota, and Wisconsin (Yang et al. 2001; Mengistu et al. 2002; Neves et al. 2020, 2022). More common observances in northern states may be explained by the combination of warm temperatures during the winter and the capability of the pathogen to survive for up to 24 months in plant residue remaining on the soil surface by the increasing use of conservation tillage practices (Mian et al. 2008; Cruz and Dorrance 2009; Zhang and Bradley 2014).
Epidemics of FLS have increased in frequency and severity worldwide, and thus have become a very important yield-reducing disease across the major soybean-producing countries. Soybean yield losses caused by FLS epidemics can range from 31% to up to 60% due to reduced photosynthetic leaf area, premature defoliation and reduced seed weight (Dashiell and Akem 1994; Mian et al. 1998). In the United States and Ontario, Canada, the estimated average annual soybean yield losses, caused by FLS, from 2010 to 2019, ranged from 101,467 to 1,453,225 metric tons (Allen et al. 2017; Bradley et al. 2021). Additionally, losses due to FLS during the 2009/10 crop season were estimated at about $2 billion USD in Argentina (Sepulcri et al. 2015). Average yields of non-protected plants against FLS were reduced by 37% in Zambia during the 1997/98 crop season (Mwase and Kapooria 2000). In Brazil, the occurrence of FLS is part of a complex of late-season diseases caused by Cercospora kikuchii, Septoria glycines, and Colletotrichum truncatum, and up to 30% yield losses have been reported (Balardin 2002).
Taxonomy
Domain Eukarya, kingdom Fungi, subkingdom Dikarya, phylum Ascomycota, subphylum Pezizomycotina, class Dothideomycetes, order Mycosphaerellales, family Mycosphaerellaceae, genus Cercospora, species Cercospora sojina (NCBI).
Identification
Morphological characterization
Although Cercospora sojina is recognized as the causal agent of FLS, early literature reported Cercospora daizu as the causal agent of this disease (Athow 1987). Conidia are hyaline, elongate to fusiform and measure 6–8 × 40–60 μm (Wise and Newman 2015). Additionally, conidia can be produced on infected parts of the plant (leaf, stem or seeds) and from infested residue on the soil surface (Cruz and Dorrance 2009). As conidiophores continue to grow, conidia are formed on the tips and are pushed aside (Groenewald et al. 2013; Wise and Newman 2015). In a single lesion, 2 to 25 conidiophores can be produced, and each conidiophore can produce 1 to 11 conidia (Lehman 1928). Conidia can germinate on a leaf surface within an hour of deposition in the presence of water at 25 to 30 °C (Phillips 1999).
Molecular characterization
Conventional polymerase chain reaction (PCR) assays have been successfully developed and used to identify and detect several important plant pathogens. C. sojina can be identified by amplifying a fragment of actin, calmodulin, histone, translation elongation factor, as well as internal transcribed spacer regions and the 5.8S rRNA gene (Groenewald et al. 2013; Neves et al. 2022). However, translation elongation factor and calmodulin genes can be intermixed with other Cercospora species (Groenewald et al. 2013). These genes can be amplified using primers (Table 1), and nucleotide sequences can be compared using the BLAST search on NCBI Genbank.
Disease symptoms
Although disease symptoms most commonly appear during reproductive growth stages, FLS lesions can affect leaves, pods and stems at any stage of development (Wise and Newman 2015). Symptoms include small, dark lesions that evolve from tan to brown spots surrounded by a narrow, purple-brown margin (Wise and Newman 2015) (Fig. 1A). The lesion diameters range from 1 to 5 mm (Grau et al. 2004). On the abaxial surface, the formation of clusters of conidia can be observed in the center of mature lesions (Wise and Newman 2015) (Fig. 1B). Stem lesions, which are two to four-times longer and wider than leaf lesions, are less common, but they can appear later in the season (Bisht and Sinclair 1985). Additionally, the fungus can penetrate through the pod walls and infect the seeds (Phillips 1999). Symptoms on seeds include light to dark gray or brown areas that can range from specks to large blotches covering the entire seed coat (Bisht and Sinclair 1985).
Disease cycle and epidemiology
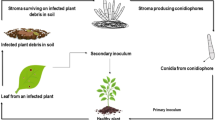
Initial inoculum can be produced on infected plant residue where the pathogen can overwinter for up to 24 months, or it can survive on infected seeds (Singh and Sinclair 1985; Cruz and Dorrance 2009; Zhang and Bradley 2014). Conidia are then dispersed throughout the crop canopy from the infested residue by wind or splashing rain. C. sojina infects the plants withbranched hyphae through open stomata. The lesions are not visible for nearly 14 days after infection. Fully expanded leaves are more resistant to infection than young expanding leaves which are highly susceptible. For plants grown in warm (25–30 °C) and humid conditions (> 90% relative humidity), sporulation can occur within 48 h of the appearance of visible symptoms (Wise and Newman 2015). Under favorable conditions, secondary infection of leaves, stems, and pods continue throughout the soybean growing season, characterizing the disease as polycyclic (Fig. 2) (Wise and Newman 2015). In seeds, the fungus can penetrate both indirectly through pores and cracks in the seed coat or directly through hilar tracheids and may grow into seedling tissues during germination and emergence (Singh and Sinclair 1985). Seed transmission can play an important role in disease spread as the disease has been found in fields never planted to soybean or under soybean rotation in Argentina, indicating that the pathogen was likely introduced via infected seeds (Sautua et al. 2018).
Disease cycle of Cercospora sojina in soybeans (Bradley et al. 2016)
Pathogenicity
Luo et al. (2018) assembled the genome of C. sojina race 1 and obtained a total assembly size around 40.84 Mb. Additionally, the genome of C. sojina contained 11,655 protein-coding genes, of which a total of 233 proteins were predicted as the putative small (400 amino acids) cysteine-rich proteins (Luo et al. 2018). The authors found 141 putative effectors and more than one third of them were upregulated during starvation suggesting that C. sojina can probably deploy effectors to promote infection (Luo et al. 2018). Despite the fact that most of the species across the Cercospora genus can produce a toxin called cercosporin, it has been disputed if C. sojina produces it (Goodwin et al. 2001). Luo et al. (2018) identified a gene cluster with eight cercosporin biosynthesis genes in the C. sojina genome and observed the increased transcription of the eight genes during infection. These results imply that C. sojina may produce cercosporin during infection. However, authors were unable to detect cercosporin in either cultured mycelium or infected plant tissue (Luo et al. 2018). Finally, in the C. sojina genome, there were around 23.5% potential secreted proteins that were predicted as putative carbohydrate-active enzymes (CAZymes), demonstrating that C. sojina may employ a large group of CAZymes to digest host cell walls during invasion (Luo et al. 2018). Another study sequenced Race 15 of C. sojina and analyzed the comparative genome with respect to Race 1 (Gu et al. 2020). The authors found that the pathogenic reaction patterns of Race 1 and Race 15 were similar.
Genetic diversity
Bradley et al. (2012) used amplified fragment length polymorphism (AFLP) markers to better understand the genetic diversity of a historical collection of 62 C. sojina isolates from Brazil, China, Nigeria, and the United States. The authors found a high degree of genetic diversity with no clear separation of isolates based on their origin. Only two isolates collected from Georgia and two isolates from China were clustered together among the two major clusters and seven sub-clusters obtained. Another study investigated the genetic diversity of a subset of 186 isolates of C. sojina, including historical isolates, which were genotyped for 49 single nucleotide polymorphism (SNP) markers, revealing 35 unique genotypes (Shrestha et al. 2017).
Sexual recombination
Sexual reproduction is a key mechanism through which genetic diversity is produced in many plant-pathogenic fungi (Glass and Kuldau 1992). Although for most Cercospora spp., including C. sojina, a sexual stage has not been observed in either field or laboratory conditions, molecular analyses have shown that Cercospora spp. form a monophyletic group within the teleomorphic genus Mycosphaerella (Goodwin et al. 2001; Crous and Braun 2003; Crous et al. 2004a). In fact, comparative genome analysis of C. sojina with plant pathogen members of the genus Mycosphaerella (M. pini, M. Populorum, Z. tritici [M. graminicola] and M. fijiensis) on different plant hosts (pine, poplar and banana, respectively) found considerable conserved synteny, higher average exon numbers per gene and gene density between C. sojina and Z. tritici compared to the genomes of the other three fungal species in the genus Mycosphaerella (Zeng et al. 2017). These genome features can be explained by the fact that the hosts of C. sojina and Z. tritici, soybean and wheat, have similar characteristics of growing conditions and pathogen resistance, compared with perennial tree species pine, poplar, and banana as hosts of M. pini, M. populorum and M. fijiensis, respectively (Zeng et al. 2017).
When the sexual stage is not known, which is the case of C. sojina, several approaches have been used to provide evidence of cryptic sexual reproduction, including quantification of genetic diversity, population differentiation, and mating-type frequencies (Kim et al. 2013). Typically, populations undergoing sexual reproduction exhibit high genetic diversity and equal mating-type frequencies compared with populations solely or predominantly reproducing asexually (Milgroom 1996). Kim et al. (2013) developed a multiplex PCR assay with specific primers for C. sojina aiming to determine mating types for a collection of 132 C. sojina isolates collected from six fields in Arkansas. Of the 132 C. sojina isolates, 68 isolates had the MAT1-1–1 idiomorph, and 64 isolates had the MAT1-2 idiomorph. No isolates possessed both idiomorphs. An equal proportion of mating-type loci in all populations analyzed and high genotypic diversity (26 to 79%) suggested that populations of C. sojina in Arkansas are most likely undergoing cryptic sexual reproduction (Kim et al. 2013). Another study investigated the genetic diversity of a subset of 186 isolates of C. sojina, including historical isolates, which were genotyped for 49 single nucleotide polymorphism (SNP) markers, revealing 35 unique genotypes (Shrestha et al. 2017). Both mating type alleles (MAT1-1–1 and MAT1-2) were found in individual lesions suggesting opportunity for sexual recombination (Shrestha et al. 2017).
Management
Host genetic resistance and races
In the United States, a total of 12 races of C. sojina were reported from various states (Grau et al. 2004) (Table 2). In Brazil, 25 races have been reported (Yorinori and Klingelfuss 1999), and in Argentina, races 11 and 12 were identified during the 2008/09 and 2009/10 growing seasons (Scandiani et al. 2012) (Table 2). In China, 11 races of C. sojina were identified and, among them, races 1, 7, and 10 were considered the major ones (Huo et al. 1988). However, the total number of races in China increased to 14 and, more recently, a race 15 was reported to be the dominant, occurring 36% more frequently than the previously dominant race 1 (Gu et al. 2020) (Table 2). This has led to a loss of host resistance in many cultivars in China (Gu et al. 2020).
Grau et al. (2004) stated that different sets of soybean differential cultivars were used to identify the C. sojina races in the United States, Brazil, and China. Additionally, Mian et al. (2008) pointed out the lack of a universally accepted set of soybean differential cultivars for the classification of C. sojina isolates into races as well as to identify, designate and compare races of this pathogen. Hence, the later authors created a new set of soybean differential cultivars and revised the C. sojina race designations to advance the characterization of C. sojina races and to identify additional FLS resistance genes in soybean (Mian et al. 2008). A total of 93 C. sojina isolates were analyzed for their reaction on 38 putative soybean differential cultivars resulting in 3,534 isolate–differential combinations (Mian et al. 2008). The authors initiated the new race structure with race 5, since there are no known existing cultures of races 1 to 4, and identified 11 unique isolates, designated races as 5 to 15 (Mian et al. 2008).
The approach used by Mian et al. (2008) does not take into account the range of disease severity reaction in each of those differentials. Therefore, Mengistu et al. (2020) proposed a new approach, known as Pathogenicity Group, to address and simplify the current system of C. sojina race designations. The authors evaluated the diversity of 83 C. sojina isolates collected from 2006 to 2009 by using pathogenicity groups among 12 soybean differentials (Davis, Peking, Kent, CNS, Palmetto, Tracy, Lincoln, S-100, Richland, Blackhawk, Hood and Lee). The set of 83 isolates grouped into five pathogenicity groups (PG1 through PG5) representing the virulence diversity present in those isolates collected from various geographical regions (Mengistu et al. 2020). PG1 did not infect eight of the differentials except Blackhawk, Lincoln, S-100, and Lee; PG2 showed low virulence on all differentials except on Davis (hypersensitive reaction); PG3 produced hypersensitive reaction on Davis but with less than moderate reaction to the rest of the differentials; PG4 caused no infection on Davis but moderate infection on Peking; and, PG5 was the most virulent pathotype that infected all genotypes except Davis (Mengistu et al. 2020). Therefore, even the most virulent pathogenicity group could not overcome the resistant Rcs3 gene in Davis and, until now, there are no Rcs3-virulent races reported in the literature. Similarly, a previous study screened 40 isolates of C. sojina collected in 2018 and 2019 across six counties in Georgia, and found no isolates pathogenic on Davis, suggesting that the Rcs3 gene is still an effective source of resistance in Georgia (Harrelson et al. 2021).
The Rcs3 gene is one of the three single dominant genes conditioning resistance to C. sojina recognized by the Soybean Genetics Committee (Mian et al. 2009). The first gene found was Rcs1 in Lincoln, which conferred resistance to race 1 of C. sojina (Athow and Probst 1952). Rcs2 was identified in Kent for resistance to race 2 (Athow et al. 1962). Finally, Rcs3 from Davis was found to condition resistance to race 5 and to all other known races of C. sojina in the United States (Phillips and Boerma 1982; Boerma and Phillips 1983) as well as to all known isolates of C. sojina in Brazil (Yorinori and Klingelfuss 1999) (Table 2). Although other dominant genes for resistance to race 5 were found in the cultivars Ransom, Stonewall and Lee in 1993 (Pace et al. 1993), they were not considered to be important sources of resistance, because, currently, race 5 is not seen as an economic threat to soybean in the United States (Baker et al. 1999). Additionally, another single dominant gene nonallelic to Rcs3 was found from the cultivar Peking and provided resistance against many C. sojina isolates (Baker et al. 1999).
In China, the gene Rcsc7 was assigned to a dominant gene for conditioning resistance to Chinese race 7 (Table 2), but it has not been officially approved by the Soybean Genetics Committee as the allelism between Rcsc7 and other resistance genes is not known (Zou et al. 1999). In Brazil, F1 plants were obtained from the diallel mating of seven soybean cultivars (Bossier, Cristalina, Davis, Kent, Lincoln, Paraná, and Uberaba), and their reactions were evaluated against C. sojina race 4 using a multivariate variable developed from soybean reactions to infection degree, mean lesion diameter, percent of lesioned leaf area, lesions per square centimeter, and disease index (Gravina et al. 2004). The authors reported that Davis, Cristalina, and Uberaba were free of FLS symptoms (Gravina et al. 2004). Mengistu et al. (2011) assessed resistance to C. sojina race 11 by field screening maturity groups I to VI across two locations (Missouri and Illinois). A total of 260 accessions including 12 differentials resulted in 20 remaining resistant accessions that might contain novel loci for FLS resistance as the presence of Rcs3 allele was not found using molecular markers (Mengistu et al. 2011).
Quantitative resistance to race 2 of C. sojina was identified in the greenhouse using recombinant inbred lines derived from the cross of the cultivars Essex and Forrest (Sharma and Lightfoot 2014). Essex is known to be partially resistant while Forrest is partially susceptible to mixed races of C. sojina. The authors inferred that quantitative resistance to C. sojina race 2 involved two major quantitative trait loci (QTL). The two loci were effective at different stages of seedling development, suggesting they were conditional QTL, and, according to the location of the QTL, the loci were not allelic to Rcs3 (Sharma and Lightfoot 2014). Recently, McAllister et al. (2021) also screened 91 recombinant inbred lines from the crossing between Essex and Forrest under greenhouse conditions for FLS resistance to C. sojina race 15 and used single nucleotide polymorphism (SNP) markers to identify associated QTL. Two QTL were mapped, being one QTL reported on chromosome 13 coinciding with the QTL previously reported (Pham et al. 2015), and the QTL on chromosome 19 was novel (McAllister et al. 2021).
Biocontrol
The use of beneficial microorganisms to control plant diseases is an alternative or a supplemental way of reducing the use of chemicals. In the U.S., Lysobacter enzymogenes strain C3 (LeC3) was tested against C. sojina, which effectively inhibited its vegetative mycelial growth and conidial germination on plates (Nian et al. 2021). Moreover, a previous study reported that the application of Trichoderma virens conidial suspensions as a foliar treatment significantly reduced frogeye leaf spot severity of soybean compared to a nontreated control (Lacey 2018). A previous study in Argentina reported reduced mycelial growth of C. sojina in vitro by testing a cell suspension of three indigenous bacterial strains, including BNM297 (Pseudomonas fluorescens), BNM340 and BNM122 (Bacillus amyloliquefaciens) (Simonetti et al. 2012). The authors found that Bacillus BNM122 and BNM340 inhibited the fungus to a similar degree (52–53%). Additionally, a significant inhibition of conidial germination was observed after 24 and 72 h of co-cultivation with cell suspension from BNM297, BNM340 or BNM122, (~ 79%, 79% and 89%, respectively). Biocontrol tests in vivo were conducted and both spray-applied bacteria, BNM340 and BNM122, significantly reduced the disease severity to a similar degree with respect to positive control plants, showing no significant differences between them, while P. fluorescens BNM297 did not affect FLS severity on soybean plants (Simonetti et al. 2012).
Induced systemic resistance (ISR) consists of the activation of a plant defense upon pathogen attack by triggering a cascade of reactions that spread from the site of induction to distant parts of the plant (Kloepper et al. 1992). Previous studies showed that soybean plants inoculated with Bacillus sp. CHEP5 had less FLS severity, with healthier and greener leaves compared to non-inoculated plants (Tonelli and Fabra 2014). Additionally, as Bacillus sp. CHEP5 was applied onto the roots and the response was detected in the shoot system, the bacterial induction of resistance in the plant was considered to be systemic, hence, attributed to ISR (Tonelli and Fabra 2014). The authors also investigated if the mechanism to induce systemic resistance of Bacillus sp. CHEP5 involved the priming of the jasmonic acid dependent pathway. The increased expression of the defense related gene GmAOS in Bacillus sp. CHEP5 plus pathogen challenged plants strongly suggest that the enhanced soybean resistance to C. sojina attack induced by this bacterium occurs in a jasmonic acid dependent pathway (Tonelli and Fabra 2014). Additionally, a following study showed a mutualistic behavior between Bacillus sp. CHEP5 with the nitrogen fixing strain Bradyrhizobium japonicum E109 being more effective in reducing frogeye leaf spot severity than the inoculation of Bacillus sp. CHEP5 alone (Tonelli et al. 2017).
Cultural practices
Cultural practices such as crop rotation and tillage can help to reduce FLS incidence (Grau et al. 2004; Wise and Newman 2015). A previous study recommended that crop rotation with a nonhost of a minimum of two years would be more effective to reduce the level of viable C. sojina inoculum, regardless of the depth of the crop residue in the soil (Zhang and Bradley 2014). Tillage can reduce the inoculum by burying infested plant residues (Mengistu et al. 2014). However, recent studies in Tennessee have not found significant differences in FLS severity, in the absence of fungicide application, between tilled and no-till plots across field trials conducted from 2007 to 2010 (Mengistu et al. 2014), and from 2014 to 2016 (Mengistu et al. 2018). Although tillage alone did not significantly affect disease, fungicide efficacy was greater in tilled compared to no-till plots (Mengistu et al. 2014). Moreover, early planting seems to be favorable to avoid higher FLS pressure, as a previous study reported higher yield reduction due to FLS when planting was delayed two weeks after the optimum planting date (Akem and Dashiell 1994).
Chemical control
The regular use of fungicides in the United States started in 2005 driven by an increase in soybean prices and the potential threat of Asian soybean rust (Phakopsora pachyrhizi) (Phillips et al. 2021). Fungicide applications aiming to control FLS are recommended during reproductive growth stages (Akem 1995). Active ingredients from different fungicide classes available for managing FLS include demethylation inhibitors (DMI), quinone outside inhibitors (QoI), methyl benzimidazole carbamates (MBC), succinate dehydrogenase inhibitors (SDHI) and chloronitriles (Crop Protection Network 2022). MBCs act in the cytoskeleton by inhibiting the formation of the β tubulin assembly during mitosis (Olaya and Geddens 2019). QoIs and SDHIs are fungicides that inhibit respiration (Sierotzki and Scalliet 2013; Fungicide Resistance Action Committee 2020). The QoIs act in the complex III in the mitochondria, binding the activity of the quinol oxidation (Qo) site of the Cytochrome b, which avoid electron transfer between Cytochrome b and Cytochrome c, interrupting ATP synthesis (Bartlett et al. 2002; Sierotzki and Stammler 2019). On the other hand, the SDHIs act in the complex II of the electron transport chain in the mitochondria, also inhibiting the production of ATP (Sierotzki and Scalliet 2013; Klappach and Stammler 2019). The DMIs are compounds that inhibit the sterol biosynthesis in membranes, which can cause cell rupture and electrolyte leakage (Mehl et al. 2019; Kumar et al. 2021). Finally, chlorothalonil is a multi-site fungicide that belongs to the chloronitriles group and is used as a protectant fungicide (Miles et al. 2007; Battaglin et al. 2011).
QoI fungicides, mainly azoxystrobin, pyraclostrobin and trifloxystrobin, have been commercially available and largely used on soybean in the United States, including for FLS management (Sauter et al. 1999; Dorrance et al. 2010; Nelson et al. 2010; Mengistu et al. 2018). However, after the emergence of QoI-resistance, studies have shown that DMIs, MBCs, SDHIs and premixes can be effective for managing FLS (Backman et al. 1979; Dorrance et al. 2010; Butler et al. 2018; Mengistu et al. 2018; Phillips et al. 2021; Viggers et al. 2022). For instance, benomyl (MBC) was very effective in reducing FLS severity among susceptible cultivars in Alabama, United States (Backman et al. 1979) and Zambia (Mwase and Kapooria 2000). However, another study conducted in Zimbabwe in 1996 and 1997 reported that the DMI flusilazole and the mixture of flusilazole + carbendazim were more effective against FLS than benomyl applied alone or as a premix with mancozeb (Galloway 2008). Recently, Mengistu et al. (2018) showed significantly higher efficacies (> 70%) for flutriafol (DMI), thiophanate-methyl (MBC) and the premix azoxystrobin + difenoconazole (QoI + DMI), compared to the single application of pyraclostrobin (27%) and chlorothalonil (30%). Additionally, a previous study summarized data from 66 uniform fungicide trials conducted from 2012 to 2021 across the major soybean-producing states in the U. S. using a meta-analytic approach (Barro et al. 2023). On average, the authors found the most effective fungicides to be the premixes difenoconazole + pydiflumetofen, thiophanate-methyl + tebuconazole, azoxystrobin + difenoconazole and trifloxystrobin + prothioconazole, all with percent control greater than 50%. The poorly performing fungicide was pyraclostrobin (11%). A statistically significant decline in performance over the years was detected for two dual premixes (azoxystrobin + difenoconazole and thiophanate-methyl + tebuconazole) and two single active ingredients (pyraclostrobin and tetraconazole), which can be linked to fungicide resistance issues (Barro et al. Unpublished).
Fungicide application timing and coverage are critical for optimal disease control. Akem (1995) evaluated applications of the fungicide benomyl at six different growth stages, starting from V3 (fully developed leaves, beginning with trifoliate nodes) to R5 (beginning seed), to determine the effect of the fungicide timing on frogeye leaf spot severity and found that applications at R1 (beginning bloom) and R3 (beginning pod) significantly reduced disease severity. Regarding coverage, Butler et al. (2018) conducted field experiments in 2014 and 2015 in Tennessee to evaluate the influence of droplet size on foliar fungicide efficacy. The authors found no significant differences among the industry recommended standard flat fan XR11002VS (XR) nozzle and the drift-reduction nozzle type TTI11002-VP (TTI) but found significant disease reduction after application of azoxystrobin + difenoconazole compared to the nontreated control (Butler et al. 2018). Additionally, results from ten field trials conducted from 2017 to 2020 in Iowa by applying fluxapyroxad + pyraclostrobin using a traditional ground sprayer with an overhead spray boom and a ground sprayer with 360 undercover sprayers showed no statistical difference between fungicide application methods on FLS severity (Viggers et al. 2022).
As mentioned previously, the primary inoculum sources of the disease are infected seeds and plant residue. Therefore, the use of pathogen-free or fungicide-treated seeds is crucial to prevent the introduction and further spread of the disease (Sautua et al. 2018). A previous study in Argentina evaluated the effect of fungicide seed treatments in reducing FLS incidence and found that premixes including benzimidazole fungicides, such as pyraclostrobin + thiophanate-methyl and carbendazim + thiram, were more effective to eradicate the pathogen in seeds (Sautua et al. 2018).
Fungicide resistance
C. sojina isolates with reduced sensitivity to quinone outside inhibitor (QoI) fungicides were first reported from Tennessee in 2010 (Zhang et al. 2012a). The resistance mechanism involved is an amino acid substitution (glycine is replaced with alanine at the codon 143) caused by the G143A mutation in the Cytochrome b gene (Zeng et al. 2015). However, other mutations associated with resistance to QoI fungicides such as the F129L (change from phenylalanine to leucine at codon 129) and G137R (change from glycine to arginine at codon 137), have not been reported in C. sojina (Zeng et al. 2015). Since the first confirmation in 2010, QoI-resistant isolates have become widespread across more than 20 soybean-producing states in the U.S. (Zhang et al. 2012a, b, 2018; Standish et al. 2015; Zeng et al. 2015; Mathew et al. 2019; Zhou and Mehl 2020; Neves et al. 2020, 2021, 2022; Harrelson et al. 2021) (Fig. 3).
States where QoI-resistant isolates of C. sojina were detected due to the amino acid substitution (glycine is replaced with alanine at the codon 143) caused by the G143A mutation in the Cytochrome b gene. Adapted from Harrelson et al. (2021), Mathew et al. (2019), Neves et al. (2020, 2021, 2022), Standish et al. (2015), Zeng et al. (2015), Zhang et al. (2012a, 2012b, 2018) and Zhou and Mehl (2020)
Several methods have been used to identify whether C. sojina isolates are sensitive or resistant to QoI fungicides. First, the effect of fungicide in vitro is a standard bioassay to evaluate the influence of chemistries and determine the effective concentration that reduces fungal growth or conidia germination by 50% relative to the non-amended control (EC50) (Fungicide Resistance Action Committee 2020). Based on EC50, the discriminatory dose assay determined for C. sojina was 1 µg/ml of azoxystrobin, 0.1 µg/ml for pyraclostrobin and 1 µg/ml for trifloxystrobin (Zhang et al. 2018). Conidia that germinated on the discriminatory dose will be considered resistant to QoI fungicides. Second, molecular methods also can be used to identify QoI-sensitive and –resistant isolates of C. sojina. Zeng et al. (2015) developed specific primers for PCR assay to recognize a mutation point that confers resistance to QoI fungicides. The primers used to identify QoI-sensitive isolates (Cs-2F/Cs-5R-2) produce a 359 bp fragment whereas the primers used to identify QoI-resistant isolates (Cs-1F/Cs-1R-2) produce a 207 bp fragment (Table 3). Additionally, Mut4-F/Mut4-R primers can amplify a fragment of the Cytochrome b gene that spans the area of F129L, G137R and C143A mutations (Zeng et al. 2015) (Table 3). Standish et al. (2015) developed a polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) to identify the G143A mutation in C. sojina using the restriction enzyme Alul. With that, PCR products from QoI-resistant isolates will produce two fragments, while QoI-sensitive isolates will remain intact upon digestion with restriction enzymes. Zhou and Mehl (2020) designed PCR (FLS-F2/FLS-R2) and pyrosequencing (FLS-S2) primers that target the presence of the G143A mutation in the Cytochrome b gene of C. sojina (Table 3).
Since QoI resistant populations of C. sojina in the United States have become widespread, growers have relied more on demethylation inhibitor (DMI) and methyl benzimidazole carbamate (MBC) fungicides, applied alone or as premixes (Zhang et al. 2021). Although DMI and MBC fungicides are classified as medium and high risk, respectively, for fungicide resistance development (Fungicide Resistance Action Committee 2020), C. sojina isolates resistant to these fungicide classes have not yet been reported in the United States. Zhang et al. (2021) investigated the sensitivity to the DMI fungicides, flutriafol and tetraconazole, and the MBC fungicide, thiophanate-methyl, for 145 C. sojina isolates collected prior to 2001 (baseline isolates), and from 2007 to 2012, representing 12 states (AL, GA, IA, IL, LA, MS, SC, WI, KY, TN, AR and NY). No shift towards reduced sensitivity to the DMI and MBC fungicides was found between baseline isolates versus isolates collected from 2007 to 2012 (Zhang et al. 2021).
Therefore, rotating modes of action that have no resistance detected yet is very important as a study reported that the application of a fungicide mix containing a QoI (azoxystrobin) and a DMI (difenoconazole) resulted in a higher proportion of resistant isolates compared to the non-treated plots, suggesting fungicide mixes can still exert selection pressure for QoI resistance (Shrestha et al. 2017).
Conclusions and future directions
In the United States, FLS is an important soybean disease that has been widespread from southern to northern states of the country, causing yield losses as well as raising fungicide resistance issues. However, knowledge of the impact of seed-borne infection on FLS development in the United States and the effectiveness of fungicide seed treatments in seed health are limited. Additionally, many questions relating to population biology, variability and pathotype structure remain unanswered, which is very important for breeding programs to successfully develop resistant cultivars. Studies explaining the mechanism of variation in this species would be extremely important in providing a deep understanding of the role of sexual recombination in creating diversity and will significantly advance our knowledge in areas relating to its pathogenicity, fungicide resistance and management.
The need to identify additional sources of resistance to C. sojina is critical as new variants have shown virulence to most soybean differentials under greenhouse inoculations (Mengistu et al. 2020). Screening in the field and/or greenhouse for evaluating accessions for reaction to C. sojina present many disadvantages such as the time required and the difficulties of inoculating with one/multiple races or relying on natural inoculum (Mengistu et al. 2011). Development of new technologies, such as molecular markers, that can assist the selection for resistance would be useful in developing FLS-resistant soybean cultivars. Moreover, detection of foliar diseases and their severity usually relies on visual assessments, which is subjective and can be influenced by the inherent knowledge and training of raters (Liu et al. 2021). The development of disease monitoring systems automatized in digital platforms can help to reduce or minimize visual bias and optimize disease assessment in the field, mainly for disease phenotyping, which can be used for genetic mapping to identify QTLs for crop genetic resistance and in breeding efforts for resistance to FLS. Liu et al. (2021) analyzed leaf images and hyperspectral reflectance data of healthy and FLS diseased soybean leaves. The models developed by the authors of the study achieved overall accuracies ranging from 91 to 97% and serve as a theoretical reference for improving disease monitoring systems (Liu et al. 2021). Additionally, McDonald et al. (2022) developed an image processing algorithm to evaluate the percent of FLS diseased leaf area and the number of lesions on a leaf. The authors reported that the automated measurement of the percent of diseased leaf area deviated from the manually measured value by less than 0.05% on average, while the automatic lesion counting deviated by an average of 1.6 lesions from the manually counted value (McDonald et al. 2022).
Finally, continued monitoring of fungicide efficacy across field trials as well as C. sojina population sensitivity to QoI, DMI, MBC and SDHI fungicides is critical to support decision making in selecting fungicides for maximizing FLS management. Grower's decisions must take into account not only technical information such as fungicide efficacy and yield return, but also profitability and strategies to mitigate fungicide resistance issues, seeking to preserve the lifetime of site-specific fungicides. Weather-based prediction models can also help build a sustainable fungicide program by applying fungicides only when needed throughout the season. A previous study in Argentina evaluated the FLS epidemic progress in six sites of the Pampas region during the 2009/2010 soybean season and calculated meteorological variables during the nine days before each field observation of disease occurrence for each site, using weather station and satellite data (Sepulcri et al. 2015). Logistic models were used to estimate probabilities of having severe or moderate to null disease. Estimations obtained by the developed model agreed with the observed epidemiological curve for one of the sites during the 2010/2011 season and coincided with the low disease presence recorded during the 2011/2012 season (Sepulcri et al. 2015). Therefore, integrating such weather-based models within a decision support tool determining fungicide spray application can be a sound basis to protect soybean plants against C. sojina.
References
Akem CN (1995) The effect of timing of fungicide applications on control of frogeye leaf spot and grain yield of soybeans. European Journal of Plant Pathology 101:183–187
Akem CN, Dashiell KE (1994) Effect of planting date on severity of frogeye leaf spot and grain yield of soybeans. Crop Protection 13:607–610
Allen TW, Bradley CA, Sisson AJ, Byamukama E, C lhilvers MI, Coker CM, Collins AA et al (2017) Soybean yield loss estimates due to diseases in the United States and Ontario, Canada from 2010 to 2014. Plant Health Progress 18:19–27
Athow KL, Probst AH (1952) The inheritance of resistance to frogeye leaf spot of soybeans. Phytopathology 42:660–662
Athow KL (1987) Fungal diseases. p. 687–727. In: Wilcox JR (ed.) Soybeans: Improvement, production, and uses. 2nd ed. Agron. Monogr. 16. ASA, CSSA, SSSA, Madison, WI
Athow KL, Probst AH, Kartzman CP, Laviolette FA (1962) A newly identified physiological race of Cercospora sojina on soybean. Phytopathology 52:712–714
Backman PA, Rodriguez-Kabana R, Hammond JM, Thurlow DL (1979) Cultivar, environment, and fungicide effects on foliar disease losses in soybeans. Phytopathology 69:562–564
Baker WA, Weaver DB, Qiu J, Pace PF (1999) Genetic analysis of frogeye leaf spot resistance in PI54610 and Peking soybean. Crop Science 39:1021–1025
Balardin RS (2002) Doenças na soja. UFSM, Santa Maria. 107
Bartlett D, Clough J, Godwin J, Hall A, Hamer M, Parr-Dobrzanski B (2002) The strobilurin fungicides. Pest Management Science 58:649–662
Barro JP, Del Ponte EM, Allen T, Bond JP, Faske TR, Hollier C, Kandel YR, Mueller DS, Kelly HM, Kleczewski NM, Ames KA, Price P, Sikora E, Bradley, CA (2023) Efficacy and profitability of fungicides for managing frogeye leaf spot on soybean in the United States: A 10-year quantitative summary Plant Disease. https://doi.org/10.1094/PDIS-02-23-0291-RE
Battaglin WA, Sandstrom MW, Kuivila KM, Kolpin DW, Meyer MT (2011) Occurrence of azoxystrobin, propiconazole, and selected other fungicides in US streams, 2005–2006. Water, Air, & Soil Pollution 218:307–322.
Bisht VS, Sinclair JB (1985) Effect of Cercospora sojina and Phomopsis sojae alone or in combination on seed quality and yield of soybeans. Plant Disease 69:436–439
Boerma HR, Phillips DV (1983) Genetic implications of the susceptibility of Kent soybean to Cercospora sojina. Phytopathology 74:1666–1668
Bradley CA, Wood A, Zhang GR, Murray JE, Phillips DV, Ming R (2012) Genetic diversity of Cercospora sojina revealed by amplified fragment length polymorphism markers. Canadian Journal of Plant Pathology 34:410–416
Bradley C, Chilvers M, Freije A, Giesler L, Mueller D, Sikora E, Sisson A, Smith D, Tenuta A, Wise K (2016) An overview of frogeye leaf spot. Crop Protection Network. https://doi.org/10.31274/cpn-20190620-013
Bradley CA, Allen T, Sisson AJ, Bergstrom GC, Bissonnette KM, Bond J, Byamukama E, Chilvers MI, Collins AA, Damicone JP, Dorrance AE, Dufault NS, Esker PD, Faske TR, Fiorellino NM, Giesler LJ, Hartman GL, Hollier CA, Isakeit T, Jackson-Ziems TA, Jardine DJ, Kelly HM, Kemerait RC, Kleczewski NM, Koehler AM, Kratochvil RJ, Kurle JE, Malvick DK, Markell SG, Mathew FM, Mehl HL, Mehl KM, Mueller DS, Mueller JD, Nelson BD, Overstreet C, Padgett GB, Price PP, Sikora EJ, Small I, Smith DL, Spurlock TN, Tande CA, Telenko DEP, Tenuta AU, Thiessen LD, Warner F, Wiebold WJ, and Wise KA (2021) Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2015 to 2019. Plant Health Progress 22:483–495
Butler S, Kelly H, Mueller T, Kruger G, Cochran A, Raper T (2018) Influence of droplet size and azoxystrobin insensitivity on frogeye leaf spot management in soybean. Crop Protection 112:149–158
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Crop Protection Network (2022) Fungicide efficacy for control of soybean foliar diseases. CPN-1019. Available at: https://doi.org/10.31274/cpn-20190620-014
Crous PW, Braun U (2003) Mycosphaerella and its anamorphs: 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1:1–571
Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ (2004a) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50:195–214
Crous PW, Groenewald JZ, Risede J-M, Hywel-Jones NL (2004b) Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50:415–429
Cruz CD, Dorrance AE (2009) Characterization and survival of Cercospora sojina in Ohio. Plant Health Progress 10:17
Dashiell KE, Akem CN (1994) Yield losses in soybeans from frogeye leaf spot caused by Cercospora sojina. Crop Protection 10:465–468
Dorrance AE, Cruz C, Mills D, Bender R, Koenig M, La Barge G, Leeds R, Mangione D, McCluer G, Ruhl S, Siegrist H, Sundermeier A, Sonnenberg D, Yost J, Watters H, Wilson G, and Hammond RB (2010) Effects of foliar fungicide and insecticide applications on soybean in Ohio. Plant Health Progress 11:31
Fungicide Resistance Action Committee (2020) FRAC code list 2020: fungal control agents sorted by cross resistance pattern and mode of action. Fungicide Resistance Action Committee. www.frac.info
Galloway J (2008) Effective management of soyabean rust and frogeye leaf spot using a mixture of flusilazole and carbendazim. Crop Protection 27:566–571
Giorda LM and Justh GR (1983) Problemas de diagnóstico relacionados con la diversificación sintomatológica en soja en la zona central de Córdoba. INTA VIII Reunión Técnica de la Soja. San Miguel de Tucumán, Tucumán (Argentina) pp: 55
Glass LN, Kuldau GA (1992) Mating type and vegetative incompatibility in filamentous ascomycetes. Annual Review of Phytopathology 30:201–224
Goodwin SB, Dunkle LD, Zismann VL (2001) Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology 91:648–658
Grau CR, Dorrance AE, Bond J, Russin JS (2004) Fungal diseases. p. 679–763. In: Boerma HR, Specht JE (ed.) Soybeans: Improvement, production, and uses. 3rd ed. Agron. Monogr. 16. ASA, CSSA, and SSSA, Madison, WI
Gravina G de A, Sediyama CS, Martins Filho S, Moreira MA, Barros EG, Cruz CD (2004) Multivariate analysis of combining ability for soybean resistance to Cercospora sojina Hara. Genetics and Molecular Biology 27:395–399
Groenewald JZ, Nakashima C, Nishikawa J, Shin H-D, Park J-H, Jama AN, Groenewald M, Braun U, Crous PW (2013) Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75:115–170
Gu X, Ding J, Liu W, Yang X, Yao L, Gao X, Zhang M, Yang S, Wen J (2020) Comparative genomics and association analysis identifies virulence genes of Cercospora sojina in soybean. BMC Genomics 21:172
Harrelson BC, Kemerait RC, Culbreath AK, Ghimire B, Li Z, Severns PM, Buck JW (2021) Assessment of quinone outside inhibitor sensitivity and frogeye leaf spot race of Cercospora sojina in Georgia soybean. Plant Disease 105:2946–2954
Huo H, Ma SM, Lu GZ (1988) The study of Cercospora sojina Hara races in Heilongjiang Province. Soybean Science 7:315–318
Kim H, Newell AD, Cota-Sieckmeyer RG, Rupe JC, Fakhoury AM, Bluhm BH (2013) Mating-type distribution and genetic diversity of Cercospora sojina populations on soybean from Arkansas: Evidence for potential sexual reproduction. Phytopathology 103:1045–1051
Klappach K, Stammler G (2019) Resistance of plant pathogens to succinate dehydrogenase inhibitors (SDHI) fungicides (FRAC code 7). In: Stevenson KL, McGrath MT, Wyenandt CA (eds) Fungicide resistance in North America. American Phytopathological Society, St. Paul, pp 85–96
Kloepper J, Tuzun S, Kuc J (1992) Proposed definitions related to induced disease resistance. Biocontrol Science and Technology 2:349–351
Kumar R, Mazakova J, Ali A, Sur VP, Sen MK, Bolton MD, Manasova M, Rysanek P, Zouhar M (2021) Characterization of the molecular mechanisms of resistance against DMI fungicides in Cercospora beticola populations from the Czech Republic. Journal of Fungi 7:1062
Lacey JV (2018) Evaluation of Trichoderma spp. as biocontrol agents for soybean diseases. Theses and Dissertations--Plant Pathology. 24. University of Kentucky
Lehman SG (1928) Frogeye leaf spot of soybean caused by Cercospora diazu Miura. Journal of Agricultural Research 35:811–833
Liu S, Yu H, Sui Y, Zhou H, Zhang J, Kong L et al (2021) Classification of soybean frogeye leaf spot disease using leaf hyperspectral reflectance Le, K.N.Q. (Ed.). PLoS ONE 16:e0257008
Luo X, Cao J, Huang J, Wang Z, Guo Z, Chen Y, Ma S, Liu J (2018) Genome sequencing and comparative genomics reveal the potential pathogenic mechanism of Cercospora sojina Hara on soybean. DNA Research 25:25–37
Mathew FM, Byamukama E, Neves DL, Bradley CA (2019) Resistance to quinone outside inhibitor fungicides conferred by the G143A mutation in Cercospora sojina (causal agent of frogeye leaf spot) isolates from South Dakota soybean fields. Plant Health Progress 20:104–105
McAllister KR, Lee YC, Kantartzi SK (2021) QTL mapping for resistance to Cercospora sojina in ‘Essex’ × ‘Forrest’ soybean (Glycine max L.) lines. Journal of Plant Breeding and Crop Science 13:14–22
McDonald SC, Buck J, Li Z (2022) Automated, image-based disease measurement for phenotyping resistance to soybean frogeye leaf spot. Plant Methods 18:103
Mehl A, Schmitz H, Stenzel K, Bloomberg J (2019) DMI fungicides (FRAC code 3): sensitivity status of key target pathogens, field versus laboratory resistance, and resistance mechanisms. In: Stevenson KL, McGrath MT, Wyenandt CA (eds) Fungicide resistance in North America. American Phytopathological Society, St. Paul, pp 51–68
Melchers LE (1925) Diseases of cereal and forage crops in the United States in 1924. Plant Disease 40:186
Mengistu A, Bond J, Mian R, Nelson R, Shannon G, Wrather A (2011) Identification of soybean accessions resistant to by field screening, molecular markers, and phenotyping. Crop Science 51:1101
Mengistu A, Kelly HM, Bellaloui N, Arelli PR, Reddy KN, Wrather AJ (2014) Tillage, fungicide, and cultivar effects on Frogeye leaf spot severity and yield in soybean. Plant Disease 98:1476–1484
Mengistu A, Kelly HM, Arelli PR, Bellaloui N, Lin B (2018) Quantifying the effects of fungicides and tillage on Cercospora sojina severity and yield of soybean. Plant Health Progress 19:226–232
Mengistu A, Ray JD, Kelly HM, Lin B, Yu H, Smith JR, Arelli PR, Bellaloui N (2020) Pathotype Grouping of Cercospora sojina isolates on soybean and sensitivity to QoI fungicides. Plant Disease 104:373–380
Mengistu A, Kurtzweil NC, Grau CR (2002) First report of frogeye leaf spot (Cercospora sojina) in Wisconsin. Plant Disease 86:1272
Mian MAR, Boerma HR, Phillips DV, Kenty MM, Shannon G, Shipe ER, Soffes Blount AR, Weaver DB (1998) Performance of frogeye leaf spot–resistant and –susceptible near-isolines of soybean. Plant Disease 82:1017–1021
Mian MAR, Missaoui AM, Walker DR, Phillips DV, Boerma HR (2008) Frogeye leaf spot of soybean: a review and proposed race designations for isolates of Hara. Crop Science 48:14
Mian R, Bond J, Joobeur T, Mengistu A, Wiebold W, Shannon G, Wrather A (2009) Identification of soybean genotypes resistant to Cercospora sojina by field screening and molecular markers. Plant Disease 93:408–411
Miles MR, Levy C, Morel W, Mueller T, Steinlage T, van Rij N, Frederick RD, Hartman GL (2007) International fungicide efficacy trials for the management of soybean rust. Plant Disease 91:1450–1458
Milgroom MG (1996) Recombination and the multilocus structure of fungal populations. Annual Review of Phytopathology 34:457–477
Mwase WF, Kapooria RG (2000) Incidence and severity of frogeye leaf spot and associated yield losses in soybeans in agroecological zone II of Zambia. Mycopathologia 149:73–78
Nelson KA, Motavalli PP, Stevens WE, Dunn D, Meinhardt CG (2010) Soybean response to preplant and foliar-applied potassium chloride with strobilurin fungicides. Agronomy Journal 102:1657–1663
Neves DL, Berghuis BG, Halvorson JM, Hansen BC, Markell SG, Bradley CA (2022) First detection of frogeye leaf spot in soybean fields in North Dakota and the G143A mutation in the Cytochrome b gene of Cercospora sojina. Plant Health Progress 23:269–271
Neves DL, Webster RW, Smith DL, Bradley CA (2021) The G143A mutation in the Cytochrome b Gene is Associated with Quinone Outside Inhibitor Fungicide Resistance in Cercospora sojina from Soybean Fields in Wisconsin. Plant Health Progress 23:241–242
Neves DL, Chilvers MI, Jackson-Ziems TA, Malvick DK, Bradley CA (2020) Resistance to quinone outside inhibitor fungicides conferred by the G143A mutation in Cercospora sojina (causal agent of frogeye leaf spot) isolates from Michigan, Minnesota, and Nebraska soybean fields. Plant Health Progress 21:230–231
Nian J, Yu M, Bradley CA, Zhao Y (2021) Lysobacter enzymogenes strain C3 suppresses mycelium growth and spore germination of eight soybean fungal and oomycete pathogens and decreases disease incidences. Biological Control 152:104424
Olaya G, Geddens R (2019) The methyl benzimidazole carbamate fungicides (FRAC code 1). In: Stevenson KL, McGrath MT, Wyenandt CA (eds) Fungicide resistance in North America. American Phytopathological Society, St. Paul, pp 29–40
Pace PF, Weaver DB, Ploper LD (1993) Additional genes for resistance to frogeye leaf spot race 5 in soybean. Crop Science 33:1144–1145
Pham A, Harris D, Buck J, Hoskins A, Serrano J, Abdel-Haleem H, Cregan P, Song Q, Boerma H, Li Z (2015) Fine mapping and characterization of candidate genes that control resistance to Cercospora sojina K. Hara in two soybean germplasm accessions. PLoS ONE 10:5
Phillips XA, Kandel YR, Mueller DS (2021) Impact of foliar fungicides on frogeye leaf spot severity, radiation use efficiency and yield of soybean in Iowa. Agronomy 11:1785
Phillips DV, Boerma HR (1982) Two genes for resistance to race 5 of Cercospora sojina in soybeans. Phytopathology 72:764–766
Phillips DV (1999) Frogeye leaf spot. Page 20. In: Hartman GL, Sinclair JB, Rupe JC (eds) Compendium of Soybean Diseases. 4th ed.. American Phytopathological Society, St. Paul, MN
Ross JT (1968) Additional physiologic races of Cercospora sojina on soybean in North Carolina. Phytopathology 58:708–709
Sauter H, Steglich W, Anke T (1999) Strobilurins: evolution of a new class of active substances. Angewandte Chemie International Edition 38:1328–1349
Sautua FJ, Scandiani MM, Gordo M, Carmona MA (2018) Detection and chemical control of Cercospora sojina infecting soybean seed in Argentina. Tropical Plant Pathology 43:552–558
Scandiani M, Ferri M, Ferrari B, Formento N, Carmona M, Luque A, Balatti P (2012) First report of races 11 and 12 of Cercospora sojina, the causal agent of soybean frogeye leaf spot, in Argentina. Plant Disease 96:1067–1067
Sepulcri MG, Moschini RC, Carmona MA (2015) Soybean frogeye leaf spot (Cercospora sojina): first weather-based prediction models developed from weather station and satellite data. Advances in Applied Agricultural Science 3:1–13
Sharma H, Lightfoot DA (2014) Quantitative trait loci underlying partial resistance to Cercospora sojina race 2 detected in soybean seedlings in greenhouse assays. Atlas Journal of Biology 3:175–182
Shrestha SK, Cochran A, Mengistu A, Lamour K, Castro-Rocha A, Young-Kelly H (2017) Genetic diversity, QoI fungicide resistance, and mating type distribution of Cercospora sojina—Implications for the disease dynamics of frogeye leaf spot on soybean. PLoS ONE 12:e0177220
Sierotzki H, Scalliet G (2013) A Review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology 103:880–887
Sierotzki H, Stammler G (2019) Resistance of plant pathogens to QoI Fungicides (FRAC code 11). In: Stevenson KL, McGrath MT, Wyenandt CA (eds) Fungicide resistance in North America. American Phytopathological Society, St. Paul, pp 97–114
Simonetti E, Carmona M, Scandiani M, García A, Luque A, Correa O, Balestrasse K (2012) Evaluation of indigenous bacterial strains for biocontrol of the frogeye leaf spot of soya bean caused by Cercospora sojina. Letters in Applied Microbiology 55:170–173
Singh T, Sinclair JB (1985) Histopathology of Cercospora sojina in soybean seeds. Phytopathology 75:185–189
Standish JR, Tomaso-Peterson M, Allen TW, Sabanadzovic S, Aboughanem-Sabanadzovic N (2015) Occurrence of QoI fungicide resistance in Cercospora sojina from Mississippi soybean. Plant Disease 99:1347–1352
Tonelli ML, Magallanes-Noguera C, Fabra A (2017) Symbiotic performance and induction of systemic resistance against Cercospora sojina in soybean plants co-inoculated with Bacillus sp. CHEP5 and Bradyrhizobium japonicum E109. Archives of Microbiology 199:1283–1291
Tonelli ML, Fabra A (2014) The biocontrol agent Bacillus sp. CHEP5 primes the defense response against Cercospora sojina. World Journal of Microbiology and Biotechnology 30:2503–2509
Veiga P, Kimati H (1974) Influência de meios de cultura e regime luminoso na esporulação de Cercospora sojina Hara. Ciência Rural 4:159–164
Viggers J, Kandel YR, Mueller DS (2022) The impact of fungicide application method on soybean canopy coverage, frogeye leaf spot, and yield. Plant Health Progress PHP-03–22–0024-RS. https://doi.org/10.1094/PHP-03-22-0024-RS.
White TJ, Bruns T, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylognetics. In: Innis MA, Gelfand DH, Sninsky JJ, White JW (eds) A Guide to Molecular Methods and Applications. Academic Press, New York, pp 315–322
Wise KA, Newman ME (2015) Frogeye leaf spot. In: Hartman GL, Rupe JC, Sikora EJ, Domier LL, Davis JA, Steffey KL (eds) Compendium of Soybean Diseases and Pests. American Phytopathological Society, St. Paul, pp 43–45
Yang XB, Uphoff MD, Sanogo S (2001) Outbreaks of soybean frogeye leaf spot in Iowa. Plant Disease 85:443
Yorinori JT, Klingelfuss LH (1999) Novas raças de Cercospora sojina em soja. Fitopatologia Brasileira 24:509–512
Zeng F, Arnao E, Zhang G, Olaya G, Wullschleger J, Sierotzki H, Ming R, Bluhm BH, Bond JP, Fakhoury AM, Bradley CA (2015) Characterization of quinone outside inhibitor fungicide resistance in Cercospora sojina and development of diagnostic tools for its identification. Plant Disease 99:544–550
Zeng F, Lian X, Zhang G, Yu X, Bradley CA, Ming R (2017) A comparative genome analysis of Cercospora sojina with other members of the pathogen genus Mycosphaerella on different plant hosts. Genomics Data 13:54–63
Zhang GR, Newman MA, Bradley CA (2012a) First report of the soybean frogeye leaf spot fungus (Cercospora sojina) resistant to quinone outside inhibitor fungicides in North America. Plant Disease 96:767–767
Zhang G, Pedersen DK, Phillips DV, Bradley CA (2012b) Sensitivity of Cercospora sojina isolates to quinone outside inhibitor fungicides. Crop Protection 40:63–68
Zhang G, Bradley CA (2014) Survival of Cercospora sojina on soybean leaf debris in Illinois. Plant Health Progress 15:92–96
Zhang G, Allen TW, Bond JP, Fakhoury AM, Dorrance AE, Weber L, Faske TR, Giesler LJ, Hershman DE, Kennedy BS, Neves DL, Hollier CA, Kelly HM, Newman MA, Kleczewski NM, Koenning SR, Thiessen LD, Mehl HL, Zhou T, Meyer MD, Mueller DS, Kandel YR, Price PP, Rupe JC, Sikora EJ, Standish JR, Tomaso-Peterson M, Wise KA, Bradley CA (2018) Widespread occurrence of quinone outside inhibitor fungicide-resistant isolates of Cercospora sojina, causal agent of Frogeye leaf spot of soybean, in the United States. Plant Health Progress 19:295–302
Zhang G, Neves DL, Krausz K, Bradley CA (2021) Sensitivity of Cercospora sojina to demethylation inhibitor and methyl benzimidazole carbamate fungicides. Crop Protection 149:105765
Zhou T, Mehl HL (2020) Rapid quantification of the G143A mutation conferring fungicide resistance in Virginia populations of Cercospora sojina using pyrosequencing. Crop Protection 127:104942
Zou J, Dong W, Yang Q, Cao Y, Chen S (1999) Inheritance of resistance to race 7 of Cercospora sojina in soybeans and RAPD tagging of the resistance gene. Chinese Science Bulletin 44:452–455
Acknowledgements
The first author acknowledges the financial support provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for scholarship.
Author information
Authors and Affiliations
Contributions
JPB performed the writing. DLN, EMD and CAB provided input on the writing. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barro, J.P., Neves, D.L., Del Ponte, E.M. et al. Frogeye leaf spot caused by Cercospora sojina: A review. Trop. plant pathol. 48, 363–374 (2023). https://doi.org/10.1007/s40858-023-00583-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-023-00583-8