Abstract
Purpose
Apigenin is a widespread phytochemical with beneficial effects on osteoblastic differentiation. However, short half-life and unstable chemical structure restrict apigenin application in bone tissue engineering applications. Here, we investigated the impact of apigenin-loaded chitosan/gelatin (Api. Cs/Gel) membranes on the osteogenic differentiation of human adipose-derived mesenchymal stem cells (hADMSCs).
Methods
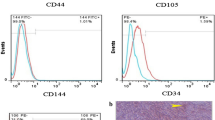
Api10. Cs/Gel and Api25. Cs/Gel membranes were fabricated using the solution casting method, followed by characterizing their physicochemical and biological properties. hADMSCs were isolated from healthy donors and characterized using flow cytometry. hADMSCs were seeded onto Api. Cs/Gel membranes and cultured under osteogenic differentiation for 7 and 21 days. The expression of osteogenic markers ALP, RUNX2, OCN, and COL1 was assessed using real-time PCR, and calcium mineralization was analyzed using the quantitative Alizarin red S staining.
Results
The Api. Cs/Gel membranes were successfully fabricated, and characterization data confirmed their structural uniformity, chemical homogeneity, cross-linking, and apigenin incorporation. Membranes exhibited favorable degradation, swelling ratio, and long-term apigenin release. The membranes were non-toxic and supported hADMSCs attachment, viability, and proliferation. The expression of ALP, RUNX2, OCN, and COL1 and cellular mineralization increased on day 21, and significant overexpression was observed in hADMSCs seeded onto Apigenin-loaded membranes.
Conclusion
Cs/Gel membranes provided an approving matrix for cellular interactions and apigenin inclusion, followed by a sustained release associated with enhanced osteogenic differentiation of hADMSCs and matrix mineralization.







Similar content being viewed by others
References
Kenkre, J., & Bassett, J. (2018). The bone remodelling cycle. Ann Clin Biochem. https://doi.org/10.1177/0004563218759371
Dan, Y., Liu, O., Liu, Y., Zhang, Y. Y., Li, S., Feng, X. B., Shao, Z. W., Yang, C., Yang, S. H., & Hong, J. B. (2016). Development of novel biocomposite scaffold of chitosan-gelatin/nanohydroxyapatite for potential bone tissue engineering applications. Nanoscale Research Letters. https://doi.org/10.1186/s11671-016-1669-1
Mizoguchi, T., & Ono, N. (2021). The diverse origin of bone-forming osteoblasts. Journal of Bone and Mineral Research. https://doi.org/10.1002/jbmr.4410
Hutchings, G., Moncrieff, L., Dompe, C., Janowicz, K., Sibiak, R., Bryja, A., et al. (2020). Bone regeneration, reconstruction and use of osteogenic cells; from basic knowledge, animal models to clinical trials. Journal of Clinical Medicine. https://doi.org/10.3390/jcm9010139
Bozorgi, A., Bozorgi, M., Khazaei, M., & Soleimani, M. (2020). Decellularized extracellular matrices in bone tissue engineering: from cells to tissues. Cell and Tissue Biology. https://doi.org/10.1134/S1990519X20060127
Black, C. R., Goriainov, V., Gibbs, D., Kanczler, J., Tare, R. S., & Oreffo, R. O. (2015). Bone tissue engineering. Current Molecular Biology Reports. https://doi.org/10.1007/s40610-015-0022-2
Bose, S., & Sarkar, N. (2020). Natural medicinal compounds in bone tissue engineering. Trends in Biotechnology. https://doi.org/10.1016/j.tibtech.2019.11.005
Dai, X., Yao, X., Zhang, W., Cui, H., Ren, Y., Deng, J., et al. (2022). The osteogenic role of barium titanate/polylactic acid piezoelectric composite membranes as guiding membranes for bone tissue regeneration. International Journal of Nanomedicine. https://doi.org/10.2147/IJN.S378422
Bozorgi, A., Khazaei, M., Soleimani, M., & Jamalpoor, Z. (2021). Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomaterials Science. https://doi.org/10.1039/D1BM00504A
Bozorgi, A., Mozafari, M., Khazaei, M., Soleimani, M., & Jamalpoor, Z. (2022). Fabrication, characterization, and optimization of a novel copper-incorporated chitosan/gelatin-based scaffold for bone tissue engineering applications. BioImpacts: BI. https://doi.org/10.34172/bi.2021.23451
Levengood, S. L., & Zhang, M. (2014). Chitosan-based scaffolds for bone tissue engineering. Journal of Materials Chemistry B. https://doi.org/10.1039/c4tb00027g
Brun, P., Zamuner, A., Battocchio, C., Cassari, L., Todesco, M., Graziani, V., et al. (2021). Bio-functionalized chitosan for bone tissue engineering. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms22115916
Thitiset, T., Damrongsakkul, S., Yodmuang, S., Leeanansaksiri, W., Apinun, J., & Honsawek, S. (2021). A novel gelatin/chitooligosaccharide/demineralized bone matrix composite scaffold and periosteum-derived mesenchymal stem cells for bone tissue engineering. Biomaterials Research. https://doi.org/10.1186/s40824-021-00220-y
Bellavia, D., Dimarco, E., Costa, V., Carina, V., De Luca, A., Raimondi, L., et al. (2021). Flavonoids in bone erosive diseases: Perspectives in osteoporosis treatment. Trends in Endocrinology and Metabolism. https://doi.org/10.1016/j.tem.2020.11.007
Weaver, C. M., Alekel, D. L., Ward, W. E., & Ronis, M. J. (2012). Flavonoid intake and bone health. Journal of Nutrition in Gerontology and Geriatrics. https://doi.org/10.1080/21551197.2012.698220
Preethi Soundarya, S., Sanjay, V., Haritha Menon, A., Dhivya, S., & Selvamurugan, N. (2018). Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2017.09.014
Bozorgi, A., Khazaei, S., Khademi, A., & Khazaei, M. (2020). Natural and herbal compounds targeting breast cancer, a review based on cancer stem cells. Iranian Journal of Basic Medical Sciences. https://doi.org/10.22038/ijbms.2020.43745.10270
Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., et al. (2019). The therapeutic potential of apigenin. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms20061305
Jung, W. W. (2014). Protective effect of apigenin against oxidative stress-induced damage in osteoblastic cells. International Journal of Molecular Medicine. https://doi.org/10.3892/ijmm.2014.1666
Pan, F.-f, Shao, J., Shi, C.-J., Li, Z.-p, Fu, W.-m, & Zhang, J.-f. (2021). Apigenin promotes osteogenic differentiation of mesenchymal stem cells and accelerates bone fracture healing via activating Wnt/β-catenin signaling. American Journal of Physiology-Endocrinology and Metabolism. https://doi.org/10.1152/ajpendo.00543.2019
Huang, Y., Zhao, X., Zu, Y., Wang, L., Deng, Y., Wu, M., et al. (2019). Enhanced solubility and bioavailability of apigenin via preparation of solid dispersions of mesoporous silica nanoparticles. Iranian Journal of Pharmaceutical Research: IJPR. https://doi.org/10.22037/ijpr.2019.2347
Soundarya, S. P., Sanjay, V., Menon, A. H., Dhivya, S., & Selvamurugan, N. (2018). Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2017.09.014
Bozorgi, A., Khazaei, M., Bozorgi, M., & Jamalpoor, Z. (2023). Fabrication and characterization of apigenin-loaded chitosan/gelatin membranes for bone tissue engineering applications. Journal of Bioactive and Compatable Polymers. https://doi.org/10.1177/08839115221149725
Stamatialis, D. F., Papenburg, B. J., Gironés, M., Saiful, S., Bettahalli, S. N. M., Schmitmeier, S., et al. (2008). Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. Journal of Membrane Science. https://doi.org/10.1016/j.memsci.2007.09.059
de la Mata, A., Nieto-Miguel, T., López-Paniagua, M., Galindo, S., Aguilar, M. R., García-Fernández, L., et al. (2013). Chitosan–gelatin biopolymers as carrier substrata for limbal epithelial stem cells. Journal of Materials Science Materials in Medicine. https://doi.org/10.1007/s10856-013-5013-3
Li, W.-y, Jia, H., Wang, Z.-D., Zhai, F.-g, Sun, G.-d, Ma, D., et al. (2020). Combinatory transplantation of mesenchymal stem cells with flavonoid small molecule in acellular nerve graft promotes sciatic nerve regeneration. Journal of Tissue Engineering. https://doi.org/10.1177/2041731420980136
Córdoba, A., Satué, M., Gómez-Florit, M., Hierro-Oliva, M., Petzold, C., Lyngstadaas, S. P., et al. (2015). Flavonoid-modified surfaces: Multifunctional bioactive biomaterials with osteopromotive, anti-inflammatory, and anti-fibrotic potential. Advanced Healthcare Materials. https://doi.org/10.1002/adhm.201400587
Jiang, L., Liu, Z., Cui, Y., Shao, Y., Tao, Y., & Mei, L. (2019). Apigenin from daily vegetable celery can accelerate bone defects healing. Journal of Functional Foods. https://doi.org/10.1016/j.jff.2019.01.043
Tungmunnithum, D., Thongboonyou, A., Pholboon, A., & Yangsabai, A. (2018). Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. https://doi.org/10.3390/medicines5030093
de Souza, M. F., da Silva, H. N., Rodrigues, J. F. B., Macêdo, M. D. M., de Sousa, W. J. B., Barbosa, R. C., et al. (2023). Chitosan/gelatin scaffolds loaded with jatropha mollissima extract as potential skin tissue engineering materials. Polymers. https://doi.org/10.3390/polym15030603
Raja, I. S., Preeth, D. R., Vedhanayagam, M., Hyon, S.-H., Lim, D., Kim, B., et al. (2021). Polyphenols-loaded electrospun nanofibers in bone tissue engineering and regeneration. Biomaterials Research. https://doi.org/10.1186/s40824-021-00229-3
Nayaka, Hanumantappa B., Londonkar, Ramesh L., Umesh, Madire K., & Tukappa, Asha. (2014). Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. International Journal of Bacteriology, 5, 35. https://doi.org/10.1155/2014/175851
Nagahama, H., Maeda, H., Kashiki, T., Jayakumar, R., Furuike, T., & Tamura, H. (2009). Preparation and characterization of novel chitosan/gelatin membranes using chitosan hydrogel. Carbohydrate Polymers. https://doi.org/10.1016/j.carbpol.2008.10.015
Ribas, R. G., Montanheiro, T. L. A., Montagna, L. S., Prado, RFd., Lemes, A. P., Bastos Campos, T. M., et al. (2019). Water uptake in PHBV/wollastonite scaffolds: A kinetics study. Journal of Composites Science. https://doi.org/10.3390/jcs3030074
Ma, H., Feng, C., Chang, J., & Wu, C. (2018). 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomaterialia. https://doi.org/10.1016/j.actbio.2018.08.026
Xie, X.-H., Wang, X.-L., Zhang, G., He, Y.-X., Wang, X.-H., Liu, Z., et al. (2010). Structural and degradation characteristics of an innovative porous PLGA/TCP scaffold incorporated with bioactive molecular icaritin. Biomedical Materials. https://doi.org/10.1088/1748-6041/5/5/054109
Tamburaci, S., Kimna, C., & Tihminlioglu, F. (2018). Novel phytochemical cissus quadrangularis extract–loaded chitosan/Na-carboxymethyl cellulose–based scaffolds for bone regeneration. Journal of Bioactive and Compatable Polymers. https://doi.org/10.1177/0883911518793913
Nakamura, T., Nakamura-Takahashi, A., Kasahara, M., Yamaguchi, A., & Azuma, T. (2020). Tissue-nonspecific alkaline phosphatase promotes the osteogenic differentiation of osteoprogenitor cells. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2020.01.136
Choi, E.-M. (2007). Apigenin increases osteoblastic differentiation and inhibits tumor necrosis factor-α-induced production of interleukin-6 and nitric oxide in osteoblastic MC3T3-E1 cells. Die Pharmazie-An International Journal of Pharmaceutical Sciences. https://doi.org/10.1691/ph.2007.3.6629
Zhang, X., Zhou, C., Zha, X., Xu, Z., Li, L., Liu, Y., et al. (2015). Apigenin promotes osteogenic differentiation of human mesenchymal stem cells through JNK and p38 MAPK pathways. Molecular and Cellular Biochemistry. https://doi.org/10.1007/s11010-015-2452-9
Bailey, S., Karsenty, G., Gundberg, C., & Vashishth, D. (2017). Osteocalcin and osteopontin influence bone morphology and mechanical properties. Annals of the New York Academy of Sciences. https://doi.org/10.1111/nyas.13470
Melguizo-Rodríguez, L., Manzano-Moreno, F. J., Illescas-Montes, R., Ramos-Torrecillas, J., de Luna-Bertos, E., Ruiz, C., et al. (2019). Bone protective effect of extra-virgin olive oil phenolic compounds by modulating osteoblast gene expression. Nutrients. https://doi.org/10.3390/nu11081722
Mroczek, J., Pikula, S., Suski, S., Weremiejczyk, L., Biesaga, M., & Strzelecka-Kiliszek, A. (2022). Apigenin modulates AnxA6- and TNAP-mediated osteoblast mineralization. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms232113179
Acknowledgements
We would like to show our gratitude to the Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran, for sharing their facilities and equipment during this research.
Funding
This work was funded by the AJA University of Medical Sciences, Tehran, Iran [Grant no IR.AJAUMS.REC.1400.301].
Author information
Authors and Affiliations
Contributions
AB: study design, experiment performance, manuscript writing, and editing. MK: technical supervision, manuscript editing. MB: experiment performance, statistical analysis, manuscript editing. ZJ: acquisition of funding; general supervision and administrative support, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki, along with the guidelines of the Ethics Committee of AJA University of Medical Sciences (Ethics no. IR.AJAUMS.REC.1400.301).
Consent to Participate
Before sample collection, informed consent was obtained from participants included in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bozorgi, A., Khazaei, M., Bozorgi, M. et al. Apigenin Release from Chitosan/Gelatin Membranes Promotes Osteogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells. J. Med. Biol. Eng. 44, 1–11 (2024). https://doi.org/10.1007/s40846-023-00832-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-023-00832-w