Abstract
The paper examines pre-combustion carbon capture technology (PreCCS) for liquefied natural gas (LNG) propelled shipping from thermodynamics and energy efficiency perspectives. Various types of LNG reformers and CCS units are considered. The steam methane reformer (SMR) was found to be 20% more energy efficient than autothermal (ATR) and methane pyrolysis (MPR) reactors. Pressure swing adsorption (PSA) had a lower energy requirement than membrane separation (MEM), cryogenic separation (CS), and amine absorption (AA) in pre-combustion carbon capture, with PSA needing 0.18 kWh/kg CO2. An integrated system combining SMR and PSA was proposed using waste heat recovery (WHR) from the engine, assuming similar efficiency for LNG and H2 operation, and cooling and liquefying of the CO2 by the LNG. The SMR-PSA system without WHR had an overall efficiency of 33.4% (defined as work at the propeller divided by the total LNG energy consumption). This was improved to 41.7% with WHR and gave a 65% CO2 emission reduction. For a higher CO2 reduction, CCS from the SMR heater could additionally be employed, giving a maximum CO2 removal rate of 86.2% with 39% overall energy efficiency. By comparison, an amine-based post-engine CCS system without reforming could reach similar CO2 removal rates but with 36.6% overall efficiency. The advantages and disadvantages and technology readiness level of PreCCS for onboard operation are discussed. This study offers evidence that pre-combustion CCS can be a serious contender for maritime propulsion decarbonization.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Shipping decarbonization has gained increasing attention in recent years following the carbon reduction goals set by the International Maritime Organization (IMO). The adoption of low- and zero-carbon fuels for ocean-going vessels has been widely discussed for its potential to reduce carbon emissions. In 2021, IMO adopted a new target to reduce carbon emission from shipping by 40% by 2030. Zero Emission Shipping Mission was launched by Mission Innovation, with the goal to operate at least 5% of global vessels by using well-to-wake (WTW) zero-emission fuels including green hydrogen, ammonia, and biofuels by 2030 [1]. Low-carbon fuels like blue hydrogen, liquefied natural gas (LNG), and methanol also have increasing demand which could help to meet the target of carbon reductions.
Each of the alternative fuel options has different energy costs, carbon reduction potentials, financial costs, and technology readiness level, which puts stakeholders in difficulty during their decision-making process. There seems to be little investment today on these alternative options. However, the uptake of LNG as a marine fuel is advancing with an increased number of newly built LNG-fuelled ships [2]. The high technological readiness level (TRL) of LNG-fuelled vessels, with effectiveness in reducing the energy efficiency design index (EEDI) and carbon intensity indicator (CII) of ships by 20%, makes LNG a promising transition fuel [3]. However, capturing the CO2 produced onboard is crucial for LNG-fuelled ships to meet IMO’s reduction goal. Hence, onboard carbon capture and storage (OCCS) is an attractive proposition [4]. Studies related to ship-based CCS have been carried out focusing on the techno-economic assessment of the technology to decarbonize ships. It was estimated that CCS installation consumed about 20% of the LNG fuel to operate solvent-based CCS units [5]. By comparing LNG with CCS installation with other alternative fuels like ammonia, methanol, hydrogen, and electricity, Li Chin et al. showed that the cost of CCS installation and energy requirement was lower than the other alternatives [5]. In addition to this, only post-engine CCS has been considered [4] [5], while pre-combustion CCS (i.e. reforming LNG to H2 and capturing the CO2) has been proposed [6] but not studied in detail yet.
Apart from LNG, the investment on the infrastructure of hydrogen is also quite notable, as evident from some ongoing large-scale projects including HySHIP [7], Topeka hydrogen project [8], and HySeas [9]. The use of hydrogen is more challenging than LNG in terms of storage due to the low energy density and extremely low boiling point (−253 °C). In terms of safety, hydrogen is less safe than LNG because the former has low minimum ignition energy, high burning velocity, and a wide flammability range [10]. In terms of cost, the cost of hydrogen per unit of propulsion energy was estimated to be three times more expensive than LNG with CCS installation due to the high hydrogen storage cost and fuel price [5] [4]. For the same propulsion energy, hydrogen needs more than 4.5 times the HFO volume; hence, for the same size of storage tank, the usage of hydrogen fuel can limit the voyage distance [5] [4]. It was also reported that the ship powered by hydrogen fuel needs to refuel three times to travel the same voyage distance as the same ship powered by HFO [4]. As highlighted by Smith et al. [11], an additional issue with liquid H2 bunkering and storage on the ship as the propulsion fuel is its higher boil-off rate as compared to LNG, which needs a reliquefaction unit that consumes a significant amount of energy. Hence, the on-shore H2 production and bunkering for use as a ship propulsion fuel incur significant penalties (energy and financial) and remain at low TRL at present.
Apart from the foreseeable impact with the usage of hydrogen fuel, another issue with the usage of hydrogen as a marine fuel is the bunkering infrastructure, which is still under development. The demand of hydrogen has grown threefold since 1975 to meet the increasing demand from oil refining and ammonia manufacturing; hence, the adoption of hydrogen as marine fuel would require hydrogen production on a larger scale to meet the bunkering demand [12]. However, using mixtures of hydrogen and LNG at different compositions can reduce the economic impact, while at the same time can prepare the ship to meet the new targets of CO2 reduction in the foreseeable future.
To resolve the above problems of hydrogen as marine fuel in terms of storage, safety, fuel availability, and low TRL bunkering infrastructure, the concept of onboard hydrogen production from LNG (see Fig. 1c, discussed later) has been introduced. Class society RINA initiated the plan to meet 70% carbon reduction target via hydrogen production [13]. Wärtsilä in collaboration with Hycamite TCD Technologies started to develop a prototype to produce hydrogen from LNG on board via methane pyrolysis process, which gives solid carbon as the by-product [14]. The commercialized “C-Zero” unit, which is a technology for the separation of hydrogen from the carbon in the natural gas, shows that there is an increasing demand for on-site production of hydrogen fuel [15]. However, the sustainability of hydrogen fuel is closely related to the production methods and operating conditions of the processes. One of the critical questions that can be raised by shipping stakeholders is which production methods and hydrogen separation technologies are more suitable for ship installation.
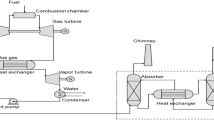
Block diagram for ship powered by combustion engine with and without carbon capture. a Conventional ship without CCS; b ship integrated with post-combustion CCS, i.e. CO2 is captured downstream of the combustion engine; c ship integrated with pre-combustion CCS, i.e. CO2 is captured upstream of the combustion engine which is using the reformer gas (mostly H2) as the fuel. For configuration, various reformers and CCS units are examined in this paper.
Previous studies on hydrogen production plants suggest that steam methane reforming reaction (SMR) has the highest efficiency of 70–85% [16, 17], followed by partial oxidation and autothermal reforming (ATR) which were claimed to have comparable thermal efficiency of 60–75% [16], water electrolysis (50–70%) [18], coal gasification (60%) [18], and methane pyrolysis (MPR) (58%) [17]. In terms of hydrogen purification (i.e. carbon capture), pressure swing adsorption (PSA) is one of the most common pre-combustion CCS (PreCCS) technologies. However, only about 52% CO2 capture rate was observed for SMR if the flue gas from the reformer is not treated, allowing about 8.2 kg CO2 emitted per kg of produced hydrogen [19] (SMR needs heat which is produced by burning methane). However, an 85% capture rate can be achieved if the reformer flue gas undergoes the post-combustion CCS [19]. In doing so, an additional 8.2% NG will be consumed [19]. Ethane pyrolysis and water electrolysis also have a considerably high carbon footprint of 4.5 kg CO2 and 17 kg CO2 respectively per kg of hydrogen produced [18]. Hence, the selection of hydrogen production methods and types of carbon capture can affect the overall efficiency of hydrogen production and the overall carbon footprint of a ship. This study aims to analyse a ship-based hydrogen production unit by integrating with a suitable type of pre-combustion CCS so that a desired carbon emission rate can be achieved at lower cost and energy consumption. The energy requirements of three methods of hydrogen production and four types of carbon capture units are quantified and compared so that a final proposal of a hydrogen production system for ships can be presented. Since the H2 production, the CO2 capture, and the H2 combustion are taking place on the same vessel, opportunities for heat integrations emerge that can reduce the overall cost of the energy-intensive H2 production and CO2 capture processes. An LNG ship carries cold fuel which can also be used to help cool and liquefy the CO2 for liquid CO2 storage. These potentials are explored in this paper so that the possibility of an onboard PreCCS system can be fully assessed.
The feasibility to use the exhaust heat for endothermic reforming has been reported [20,21,22,23,24]. As justified in Ref. [25], high-temperature exhaust gas from the engine can be considered a high-grade heat source which can reduce onboard fuel consumption by about 10%. Pashchenko et al. emphasized the concept of thermochemical exhaust heat recuperation for SMR and steam generation which can improve the overall efficiency by 4–7% [20, 23]. The heat from the SMR furnace and exhaust gas was shown feasible to pre-heat the natural gas to 773 K prior to entering the reformer; besides, the MDEA-based pre-combustion CCS was claimed to operate with almost no extra cost by utilizing the low-grade heat downstream the WGS reactors [22]. In another way of waste heat utilization, previous researchers [21, 24, 26] studied the performance of exhaust reformers by integrating the heat from the exhaust gas downstream of NG marine engine for the production of hydrogen-rich stream. It was also shown that the heat from the reformer and WGS could be recovered to pre-heat the natural gas and water for steam generation [27]. These studies demonstrated the feasibility of heat integration to reduce the energy intensity of hydrogen production and carbon capture. However, among the choices of hydrogen production methods and carbon capture technologies, which pathway performs the best? Which combination of technologies can give the best performance when being used onboard, given the particular nature of a typical ship engine? A ship-based assessment on various hydrogen production and carbon capture pathways is essential in order to bridge the existing research gap in ship-based hydrogen production.
The novelty of this study is an analysis of the concept of onboard decarbonization with pre-combustion carbon capture and with a special focus on heat integration and optimization. In the next section, the research flow and details of process simulation are presented. Section 3 includes a comparison of the alternative H2 production systems, the alternative CCS systems, and an optimized system based on SMR-PSA. Section 4 discusses the results in view of their potential for development into a realistic onboard marine system. Section 5 summarizes the most important conclusions.
2 Methods
2.1 Summary of the Calculations
Previous work [4, 5] suggested hydrogen and onboard CCS installation as potential approaches for shipping decarbonization. Here, the combination of onboard hydrogen production and CCS is investigated in more detail. Due to the difference in the performance of the existing technologies for hydrogen production, a comparative study between various technologies is necessary, and a quantification on the energy requirement helps to reflect the feasibility of hydrogen production onboard a ship in terms of energy and CO2 reduction. In this paper, we compare the overall efficiency of (a) the conventional, unabated ship (Fig. 1a); (b) a post-combustion (post-engine) CCS system based on amine absorption (Fig 1b); (c) various pre-combustion CCS systems (Fig. 1c), exploring different H2 production and carbon capture methods; and (d) an optimized, integrated steam reformer/water-gas shift/pressure swing adsorption system using waste heat recovery (Fig. 3, discussed later). These specific options were selected for optimization because, as will be demonstrated later, they offer the smallest energy penalty for capturing carbon. Figure 2 demonstrates schematically the methodology used and the various reactor options studied. More details follow next.
Figure 2 shows schematically the various components of the calculation. Thermodynamics modelling with Aspen HYSYS for different stand-alone hydrogen production and carbon capture systems was first done. Next, the hydrogen production models were optimized for maximum hydrogen production rate. The energy requirements of various reactors for optimal hydrogen production were then reported in terms of kWh/kg CH4 feed. The mass and energy balance data obtained from the Aspen simulation models were important inputs for energy and GHG assessments so that the energy efficiency (εo), CO2 reduction efficiency (βo), and hydrogen yield (αH2) could be estimated. These metrics are defined as follows: (a) εo is the measure of the efficiency based on the ratio of engine propulsion work to the overall energy input by the total LNG consumption, (b) βo is the ratio of CO2 captured to the total CO2 emission if no CCS were installed, and (c) αH2 is the ratio of the hydrogen produced to the maximum possible theoretical production of hydrogen if 100% of the methane feed were converted.
Next, the validated CCS Aspen HYSYS models that were built according to the current state of the art of carbon capture technologies were connected with the product stream from the selected reactor, and hence, the energy requirement of different CCS technologies was obtained in units of kWh/kgCO2 captured. From these results, a suitable reactor-CCS-integrated system was selected.
The selected reactor-PreCCS-integrated system was combined with the engine model in Aspen HYSYS. With this, an integrated Aspen model for hydrogen production onboard of ship was produced. The operating conditions of the integrated system were optimized with the integration of waste heat. These parameters were (a) the fraction of methane feed that is fed to the SMR furnace (XCH4) for the generation of heat for the reforming reaction; (b) the steam to methane ratio (S/C); and (c) the exit temperature of the SMR reactor (TSMR).
Finally, the possibility of using this integrated system for partial reforming, so that the engine is burning some LNG directly and some H2 produced from the reformer, was investigated to examine whether partial decarbonization can be achieved and what would be the associated energy efficiency and carbon emission.
2.2 Process Simulation of the Various Sub-systems
2.2.1 Hydrogen Production
Aspen models for steam reforming (SMR), autothermal reforming (ATR), and methane pyrolysis (MPR) were constructed. The hydrogen production from liquid methane feedstock via steam reforming and autothermal reactions was modelled as Gibbs reactors, whereby the thermodynamics of the reactions under different operating temperatures and pressures were predicted by using Gibbs free energy minimization method with the Peng-Robinson property package. MPR was modelled as a plug flow reactor using chemical reactions and kinetic parameters taken from Ref. [28]. In the SMR and MPR, some methane needs to be combusted to provide heat for the endothermic reactions. Therefore, the CH4 processing was modelled using two separate reactors: one was for methane reforming or decomposition and another was the furnace (heater) for methane combustion to provide the required heat. The CO2 emitted by this process was considered separately. For SMR and ATR, high-temperature and low-temperature water-gas shift reactors (HTWGS and LTWGS) were modelled as Gibbs reactors downstream of the steam reforming reactor unit so that the CO in the product stream was shifted into more hydrogen and CO2.
By using experimental or plant data, the Aspen models for the various types of reactors were validated. The SMR model was validated against the experimental data in [29], the ATR model was validated against the BV model presented by De Groote and Froment in Ref. [30], and the MPR was modelled based on the tubular reactor developed from experimental data as published in [28]. The validated models were then used to simulate the hydrogen production process from the feed as described in Table 1 part A. The operating parameters of the reactors as listed in Table 1 part B were manipulated and optimized by using Aspen for a maximum methane conversion and optimal hydrogen production.
Additional unit operations and energy streams were included. For electricity, the power consumptions for pump (PH2O,pump), methane compression (Pcomp), and oxygen separation (PASU) were obtained from the model of pump and compressors. In terms of heat utility requirement, heat input for steam generation (Qh,steam) and reactor heat requirement (Qh,SMR) and heat provided by combustion of methane (Qh,CH4) were obtained from the energy streams.
2.2.2 Carbon Capture and Storage (CCS)
As CO2 reduction was the main goal of this study, CCS was integrated with the reactor to produce a low-carbon hydrogen stream as the engine feed. For MPR, solid carbon was separated by using a cyclone separator, no heating or power supply was needed, and this separation process did not reduce the overall efficiency of MPR. The shifted syngas downstream of the WGS reactors for SMR and ATR reactors needed to undergo CO2 separation. In this study, four types of CCS were investigated. These were pressure swing adsorption (PSA), membrane separation (MEM), cryogenic separation (CS), and amine absorption (AA). A comparison of these four types of CCS was performed.
First, Aspen models for PSA, MEM, CS, and AA were constructed and validated. The AA simulation model was based on the optimized blended amine model presented in Ref. [31]. MEM was modelled as a two-staged membrane with CO2-selective membrane as the first stage membrane to separate CO2 as the permeate, followed by H2-selective membrane, a MOFs-polymer mixed matrix membrane characterized by [32] with a high H2 permeance of \(33\times {10}^{-9}9\ \frac{mol}{s. Pa.{m}^2}\), and H2/CO2 selectivity of 53.1. PSA was modelled as a single-stage adsorption tower with activated carbon as the adsorbent due to its high selectivity towards CO2. Finally, CS was modelled by distillation columns integrated with propane-ethylene cascade refrigeration cycle as proposed in Ref. [33].
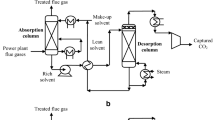
To provide a comparison between the pre-combustion CCS studied here and the post-combustion (post-engine) CCS studied in Refs [4, 34,35,36,37], the same CCS models were used for the engine exhaust gases. The differences in feed composition and operating conditions gave rise to the differences in the energy requirement and carbon capture efficiency of the CCS, which are hence worth quantifying.
The CO2 processing for onboard storage was also modelled downstream of the CCS. The typical storage condition for transportation of CO2 in liquid form was applied in this study, which was at a temperature of −20 °C and at 20 bar [38]. Hence, in the modelling of CO2 storage system, a compressor was used to compress the captured CO2 to 20 bar, followed by seawater cooling, and finally, the cold energy from regasification of LNG was used to liquefy the CO2 at −20 °C.
The energy consumption in the various reactors was grouped into electricity and heat utilities in units of kWh per kg of captured CO2. The electrical power consumption for the various types of CCS was different: PSA and AA required compression power for the captured CO2 (PCO2,comp); MEM required additional power for recompression of the permeate hydrogen before feeding to the combustion engine (PH2,comp); and CS required high compression duty to pressurize the feed to 110 bar (PHP,comp) and for compression of the propane and ethylene refrigerants in the cascade refrigeration cycles (PR,Ethylene and PR,Propane). Next, in terms of heat utilities, the only CCS that consumed heat was the AA unit for the amine regeneration (Qh,CCS). From the total power (∑P) and total heat (∑Q), the total energy consumption (∑E ) for the various CCS options was estimated and divided with the total amount of CO2 captured so that energy requirement was expressed in terms of total kWh/kgCO2 captured. With this, the energy intensity of various CCS units was compared and analysed numerically and the most suitable CCS for pre-combustion and post-combustion capture was identified.
2.2.3 Combustion Engine (ICE)
We assume that the ship is powered by an internal combustion engine with 48% of energy conversion efficiency (εEngine), representative of a low-speed large marine engine [39]. A simplified engine model with fixed efficiency of 48% was modelled in Aspen. The engine was modelled as a simplified combustion reactor integrated with air compressor and gas turbine for power generation. The exhaust gas condition was assumed with a constant pressure of 1 bar. By using the same engine in the Aspen model, an engine with the usage of methane, hydrogen, or a mixture of methane and hydrogen as marine fuel was simulated. The combustion of LNG and hydrogen gave different compositions of flue gas. The validated engine model was then used for the study of potential heat recovery from the exhaust which was different from the composition of feed fuels. The exhaust from the engine was set at T = 706.15 K.
2.2.4 Waste Heat Recovery (WHR)
In the previous steps, the energy requirement of various processes (reactor, CCS) was categorized into heat and electricity requirements. Here, WHR was integrated for the optimization of the SMR-integrated system. As will be shown later, the SMR was the most energy-efficient hydrogen production technology, so this system was selected for optimization and integration. In this study, the heat recoverable was used as heat for steam generation (Qh,steam) and heat for endothermic methane reforming (Qh,SMR). The steam was generated at 503 K, and hence, the exhaust (T ≈ 706 K) and product stream from HTWGS (T > 773 K) were used for the steam generation. The product stream from the SMR reactor (1073 K < T < 1473 K) was used for the pre-heating of methane upstream of the SMR reactor. A temperature below 503 K was assumed as heat loss (Qloss) to the surroundings that could not be further used. The amount of heat recoverable from the exhaust and high-temperature product streams was also estimated using Aspen.
Another potential heat recovery is the heat released from the liquefaction of captured CO2, which was used for methane gasification. The cryogenic LNG (111.15 K, 1 bar) was vaporized with the heat released from CO2 liquefaction for storage under 20 bar, 253 K. In this study, seawater was the main cooling medium to cool any streams to 303 K or higher as per process requirement; hence, there was a continuous supply of cooling utilities for ship.
2.2.5 Integrated System
As shown in Fig. 3, the SMR-based integrated system combined the SMR-WGS reactors, PSA, combustion engine, and the storage system for the captured CO2. The high-temperature stream from the SMR product was used for pre-heating the methane feed so that the heat requirement for reforming (Qh,SMR) could be reduced. In Aspen, the heat recoverable from the SMR product was modelled as an energy stream QWHR,SMR. The total required heat Qh,SMR was higher than the QWHR,SMR; hence, extra heat was supplied by direct combustion of some methane in the SMR furnace. The fraction of the total methane flow to be combusted as fuel (XCH4) was calculated by using Eqs. (1), (2), and (3), and the heat provided from combustion of methane is represented by Qh,CH4.
Next, the high-temperature streams from the HTWGS reactor and exhaust were used for steam generation. These streams were inter-connected into a heat exchanger so that the heat could be transferred to heat up and vaporize the cold water feed. The steam was then connected to the reformer for steam methane reforming reaction, while unused steam in the synthesis gas was sent to the HTWGS and LTWGS so that the CO product could be shifted to produce more hydrogen. Next, the shifted syngas produced by the LTWGS was fed into the CCS for the separation of CO2. The captured CO2 was compressed and cooled by utilizing the cold energy from the LNG which was stored under cryogenic condition. In Aspen, the heat transfer between the cold LNG and CO2 was modelled as a heat exchanger. The hydrogen-rich stream separated from the CO2 was fed into the combustion engine for the generation of propulsion energy. Combustion of the hydrogen-rich stream produced a high-temperature exhaust stream, which was one of the high-temperature streams used for steam production.
2.3 Metrics for Assessment
2.3.1 Hydrogen Yield (α H2)
Mass balance data was obtained from the Aspen simulation; for example, the product from the reactor as tabulated in Table 3 part A was important for the determination of the hydrogen production yield as defined by Eq. (4). The maximum theoretical mass flow of hydrogen from the reactor was estimated as per the stoichiometric Eq. (5).
2.3.2 Energy Efficiency (ε)
The energy efficiency of the base case was 48%, i.e. a propulsion output of 6.67 kWh/kgCH4 was the reference of comparison. Firstly, the electricity and energy requirements of various processes were acquired from Aspen. The electricity needs were assumed to be generated by methane also at the same conversion efficiency of 48%. The heat utilities were supplied from the direct combustion of methane fuel. The summation of total electricity (∑P) requirement and total heat (∑Q) requirement gave the total energy (∑E) which came from LNG consumption as per Eq. (6).
Due to the variation in the hydrogen production rate for the different reactors and CO2 capture rate in the CCS, all the energy consumptions (∑P, ∑Q, and ∑E) were expressed in terms of kW/kg CH4 feed to allow a direct comparison. For the hydrogen production system, the efficiency of the reactors was estimated with Eq. (7). Then, for the other systems, overall efficiency was used as an indicator of system efficiency. Part of the engine output was supplied to the electricity consumers in the integrated system: methane compressor, water pump, and CO2 compressor. The total electricity consumption was indicated as Pelec. The total propulsion output (Wprop) of the ship was calculated from Eq. (8), whereby the enthalpy of the engine feed (HICE, feed) was multiplied with 48% (the assumed engine efficiency, εEngine) and with the deduction of Pelec. Next, overall efficiency (εo) was estimated by dividing with the enthalpy of methane feed as per Eq. (9).
2.3.3 Carbon Capture Rate
The carbon capture rate is an important indicator of the system’s performance. The CO2 capture rate of the integrated system was calculated by using Eq. (10), whereby the total amount of captured CO2 was divided by the total CO2 emission for the base case. Theoretically, the CO2 emission from the base case without CCS was estimated to be equal to 274 kg/h of CO2 for 100 kg/h of methane feed, or 2.74 kgCO2/kg CH4.
2.3.4 Analysis of Variance (ANOVA) of the Optimized Integrated System
The integrated system with WHR had increased complexity. The overall energy efficiency of the integrated system was improved with waste heat recovery from high-temperature streams (i.e. the product streams from the reactors and the engine exhaust). These heats were inter-connected within the Aspen model, so an Aspen nested case study was carried out to simulate the variation of operational parameters, XCH4, S/C, and TSMR, towards the response of the integrated system (εo, βo, and αH2). The nested case study was done following the stated range and step sizes for various parameters as shown in Table 2. There were 378 simulation runs in total.
Next, the result obtained from Aspen was analysed with ANOVA, so that the interactions between the three operational parameters and the performance indicators (εo, βo, and αH2) could be estimated quantitatively. Then, optimization was performed by using the ANOVA model, so that the operating conditions for optimal εo and βo can be identified.
2.3.5 Partial Decarbonization Trajectories
The possibility that not all the engine feed was reformed was also explored by considering the case where some LNG went through the reformer and CCS, while some LNG was combusted directly in the engine without CCS. This would allow a partial decarbonization strategy that can evolve with time to meet the IMO 2030 and 2050 targets. The percentage of total LNG fed for reforming (to provide both the heat needed for the reforming and the CH4 to be reformed) was denoted by Xf,SMR, and the overall efficiency and CO2 removal were calculated as a function of this fraction.
2.3.6 Economic of Hydrogen Production
In this study, the hydrogen-rich product was produced onboard of a ship via LNG reforming. The economics of the ship-based produced hydrogen was compared with the market price of hydrogen. Here, the operating cost of the reformer-integrated system was estimated with the aid of Aspen Process Economic Analyser (APEA), and then annualized capital cost (ACAPEX) was estimated based on H2A-Lite Model [40] as per land-based hydrogen production system with pre-combustion CCS. The CAPEX cost of equipment was determined for a ship with a rated power of 15,310 kW and an operational life of 20 years. By doing so, the total production cost of hydrogen onboard of ship (CT) was estimated from Eq. (11) and expressed in the unit of $/kgH2 produced. ACAPEX and total OPEX per annum (kWh/year) were summed and divided by the total amount of hydrogen produced per annum so that the total production cost per kg of hydrogen produced could be calculated. From the literature, the cost of blue hydrogen could range from $1.5 to $4 depending on the methane cost, whereas the cost of green hydrogen costs between $2.5 and $6 [41]. With this, the economics of onboard fuel production could be assessed.
3 Results
3.1 Selection of Hydrogen Production System
Table 3 part A shows the composition of the product stream from SMR, ATR, and MPR per kg of CH4 feed. Table 3 part B shows the equivalent energy consumptions. SMR (71.53%) was the most efficient method of hydrogen production, followed by MPR (52.04%) and finally ATR (51.66%), which was in agreement with the overall efficiency reported in the literature [16, 17]. ATR showed the lowest overall efficiency due to the highly energy-intensive ASU unit that consumes high power for oxygen separation. As shown in Table 3 part B and Fig. 4, the power consumption of ATR was more than seven times the SMR power consumption. This explains the low energy efficiency of ATR and suggests that ATR may not be suitable for ship-based hydrogen production as power utilities were limited for ship. The efficiency of MPR was estimated to be 19.5% lower than SMR, attributed to the energy loss in the form of carbon by-product. MPR combusted 7.5% of the CH4 feed for heat generation, and approximately 45% of the product’s enthalpy from the MPR reactor was lost as carbon product. Due to the energy advantage of the SMR, this was selected for further studies of onboard hydrogen generation.
3.2 Comparative Study of Pre-combustion CCS and Post-combustion CCS
Table 4 parts A and B show the energy requirement of PSA, MEM, CS, and AA per 1 kg of captured CO2 so that the energy of carbon capture can be compared fairly. From Table 4 part A, the PSA was the least energy-intensive pre-combustion CCS. It consumed 0.18 kWh per kg of captured CO2, followed by membrane separation (0.59 kWh/kgCO2), amine absorption (1.14 kWh/kgCO2), and lastly cryogenic separation (1.40 kWh/kg CO2). The PreCCS feed was the gas from the SMR-WGS at high pressure (25 bar), which provided a favourable condition for the CO2 separation via a different process. Under high pressure, feed compression duty can be avoided for PSA and membrane separation, and the compression duty for the cryogenic separation was also reduced. High pressure also reduced the amount of amine solvent requirement with an increased absorption efficiency. Therefore, the regeneration duty for pre-combustion amine absorption was lower than PostCCS, which occurred at low pressure. In addition, the CO2 compression duty for CO2 was also largely reduced. Among all post-combustion capture systems, amine absorption was the least energy intensive with a consumption of 1.33 kWh/kg CO2, followed by PSA (2.29 kWh/kg CO2), cryogenic separation (2.99 kWh/kg CO2), and membrane separation (3.81 kWh/kgCO2), as shown in Table 4 part B.
The total energy required to capture 1 kg of CO2 via PreCCS was approximately 8%, 15%, 47%, and 86% of the total energy required by the PostCCS when PSA, membrane, cryogenic separation, and amine absorption were used respectively. Hence, by comparing the total energy requirement for PreCCS and PostCCS, PreCCS seems to be more energy efficient than post-combustion, with PSA being the least energy-consuming option.
3.3 Selection of Integrated Hydrogen Production-CCS System
Table 5 and Fig. 5 give the results for the SMR-CCS-integrated system. SMR without CCS gave an overall efficiency of 34.34%, and integration of CCS reduced the energy efficiency further. SMR-PSA (33.42%) was the most efficient integrated system, followed by SMR-MEM (31.55%), SMR-AA (28.36%), and lastly SMR-CS (26.53%). This is due to the energy consumption by the CCS which was lowest for PSA, followed by MEM, AA, and CS. Hence, SMR-PSA was the most energy-efficient integrated system.
However, a comparison between SMR and the best pre-combustion CCS and the base case of LNG fuel in the engine with AA post-combustion installation shows that LNG-AA installation was less energy intensive, with 36.62% energy efficiency (see Table 5 part B) as compared to the 33.42% efficiency of SMR-PSA. This can be explained by the highly energy-intensive steam reforming reaction. Hence, post-combustion CCS installation was a more energy-efficient carbon reduction approach for ship, based solely on the outcome of the energy requirements of the reactors. However, the significant heat requirement for the SMR-WGS system allows the possibility of effective waste heat utilization, and this is discussed in the next sub-section.
3.4 Optimization of Integrated SMR-PSA with Waste Heat Recovery (WHR)
Figure 6 shows the results of the optimization process, i.e. the selection of XCH4, S/C, and TSMR so as to maximize overall efficiency. Considering energy optimization, it was found that the lowest XCH4, S/C, and TSMR gave the highest efficiency. Figure 6 indicates the point with the highest εo reaches 43.2%, which occurred when XCH4 = 15%, S/C = 2, and TSMR = 800 °C. At this operating condition, the βo was very low at 41.4%. The optimization of βo showed that the highest βo was achieved at a high XCH4 (25%) so that the energy recovered was fully utilized and the additional energy requirement was supplied via combustion of extra methane feed. The point with the highest βo (66.3%) gave a relatively low energy efficiency of 40.9%. From this, we can conclude that there is a trade-off between εo and βo. Finally, examination of the integrated system for optimal εo and βo suggested to trade off the highest εo and βo with optimal εo and βo. The box in Fig. 6 indicates the zone with optimal εo and βo, with εo ranging between 40 and 42% and βo ranging between 58 and 63%.
ANOVA optimization suggested the operational parameters XCH4, S/C, and TSMR to be set at 23.68%, 3.163, and 1072 °C. At these conditions, the εo and βo increased to 41.7% and 64.9% respectively. εo increased by 24.78% with WHR, whereas the βo also increased from 55.53 to 64.9%, showing a 16.87% increment. Hence, WHR was proven to be highly effective in reducing the energy requirement and increasing carbon reduction. The WHR reduced the amount of XCH4 requirement when some of the heat can be recovered from other processes so that more methane can be fed into SMR for reforming. This resulted in a higher αH2. At the same time, the enthalpy of the SMR product or engine feed (HICE,feed) was higher and εo was improved. Also, more CO2 was captured by the PSA, which improved the βo of the integrated system.
In short, the energy efficiency of the optimal SMR-PSA with WHR (41.70%) was higher than the base case ship with amine-based post-combustion CCS, LNG-AA (36.62%). This suggests that the SMR-integrated system with WHR can have a high energy efficiency and good carbon removal rate. A proper design of the waste heat networks is therefore vital for the improvement of the overall energy efficiency.
3.5 Integrated System in Partial Decarbonization Trajectory
It is instructive to also consider partial decarbonization so that the engine is fed by a mixture of LNG and a reformate. A simplified block diagram of this dual-fuel system is shown in Fig. 7. The blocks coloured in blue show the reformer-PreCC system.
Figure 8 shows this scenario in terms of overall efficiency and carbon removal rate as a function of the fraction of CH4 that is fed to the reformer system (and hence CCS). Hence, Xf,SMR = 0% is today’s situation where the engine uses 100% LNG, while Xf,SMR = 100% is when the engine used only the reformate gas. It is evident that by increasing Xf,SMR, the degree of decarbonization increases. The overall efficiency penalty to achieve 65% carbon removal is about 7 percentage points.
Figure 9 is plotting the same results, but now with the addition of a second CCS unit based on amine absorption to capture the CO2 emissions from the CH4 burning for the SMR. Although this could be practically complicated for implementation, this calculation is included here for completeness. The βo is now maximized to 86.3% and the energy efficiency is reduced to 39%. The maximum βo of SMR-integrated system is slightly lower than the base case with post-combustion CCS (βo = 90%). However, the εo of the SMR-integrated system (εo = 39%) is about 6% higher than LNG-CCS (εo = 36.6%).
Overall energy efficiency (εo) against CO2 reduction rate (βo) for the base case (no CCS; squares), reformer-PreCCS system at the design engine exhaust temperature of Tex = 433 K, reformer-PreCCS at various exhaust temperatures (550 K < Tex < 900 K), and a reference point of post-engine CCS with βo = 90% (star). The blue upright triangles show the case where amine CCS is also included to capture the SMR heater CO2 emission
Figure 9 shows the changes in the εo and βo for the reformer system when the exhaust temperature (Tex) was varied. It was discovered that a higher exhaust temperature improved the εo and βo for the system. When Tex was low, additional methane was burnt to generate heat for steam generation as the heat recovered from the exhaust stream was insufficient. At a high Tex, more heat can be recovered from the exhaust stream which allows the generation of steam with higher temperature, which indirectly reduces the Qh,SMR requirement, and hence, a lower Qh,CH4 is needed for the reformer.
The decarbonization timeline as shown in Fig. 9 shows that both IMO2030 and IMO2050 goals can be met with either an SMR-PSA integrated pre-combustion CCS system or a post-combustion CCS system. However, the pre-combustion CCS systems exhibit better overall efficiency, suggesting that the proposed system has a promising outlook for shipping decarbonization.
3.6 Economic of Hydrogen Production
The economics of hydrogen production was projected by using the optimal model of SMR-PSA with WHR (εo = 41.70%) discussed in Section 3.4. A ship with a rated power of 15,310 kW and energy efficiency of 41.70% required 63,407 kgCH4/day (880,729 kWh/day to produce approximately 26,072 kgH2/day). Based on this hydrogen production rate, the total installed CAPEX was estimated using H2A-Lite Model to be $249,246,259. Assuming that a ship was designed to operate for 20 years, the ACAPEX was $12,462,313/year or $1.31/kgH2. The OPEX cost was estimated based on the total consumption of methane feedstock, as electricity and heat utilities were both obtained from methane feed for a ship-based operation. For production of 26,072 kgH2/day, 63,407 kgCH4/day was consumed. Based on the selling price of methane of $239/tons CH4 [42], the OPEX cost was calculated to be $5,531,344/year or $0.58/kgH2. Hence, the total cost of 1 kg of blue hydrogen produced onboard of ship with SMR-PSA is $1.89.
4 Discussion
This study has introduced a new concept for onboard carbon capture for LNG-fuelled shipping by combining two energy-intensive systems, hydrogen production, and onboard carbon capture, with effective heat integration from the LNG and the engine exhaust. The performance of the proposed system was measured in terms of overall energy efficiency (εo) and carbon capture fraction (βo). The combination of SMR and PSA was proposed as SMR was more energy efficient than ATR and MPR (Table 3 and Fig. 4), and as PSA had the lowest energy consumption compared to alternative CCS methods (MEM, AA, and CS; see Table 4). The integrated system SMR-PSA resulted in a significant reduction of overall efficiency from 48 to 33.42% (see Table 5). However, optimization with WHR improved the energy efficiency of the system to achieve εo = 41.7%. Therefore, this reformer system was able to reduce 65% of carbon emission with an energy penalty of about 7 percentage points.
Compared to post-engine CCS, which is the usual shipping decarbonization proposition based on CCS, a reformer system produces syngas with a higher concentration of CO2, which eases the carbon capture process, especially for a ship-based CCS with space constraints. Handling a smaller flow of syngas fuel than the much larger flow of flue gas produced downstream of the engine is highly favourable as this would be able to reduce the CCS size and minimize the ship space for accommodation of the CCS unit. It is also expected to have lower capital cost. In addition, a PSA-based CO2 capture process can reduce the risk and hazards associated with chemical handling and storage onboard. An additional advantage of the reforming and pre-combustion CCS is that it can also be considered “onboard blue hydrogen production” which eliminates the need to have a separate LH2 bunkering infrastructure and onboard LH2 tanks. This implies that the problem of large LH2 boil-off is eliminated and should result in a lower financial burden. Further, hazards associated with hydrogen handling are probably easier to manage, since the path from production to utilization is much shortened compared to land-based hydrogen production, bunkering, and use on the ship. Therefore, if blue hydrogen production is to happen, the present results suggest it is advantageous to do this onboard rather than on land.
The reformer system being proposed in this study consists of steam methane reformer and PSA. Globally, 95% of the hydrogen is produced from the SMR process. PSA is also a commercialized carbon capture technology with high TRL, widely used by the hydrogen industry players like Honeywell and Linde [43, 44]. So, the proposed system, which is an integration of two matured technologies, should be possible to achieve. From an economic perspective, the cost of 1 kg of blue hydrogen produced onboard of ship with SMR-PSA was estimated to be $1.89. As compared to the current price of blue hydrogen fuel which ranges from $1.5 to $4/kg [45], in situ production of hydrogen from methane is within the range of the average market price of hydrogen. In addition, the boil-off rate of hydrogen is almost 9 times higher than LNG ship [11], and then as much as 45% of the purchased liquefied hydrogen was reported to loss during fuel transportation and bunkering [46]. With such high amounts of fuel loss, the OPEX cost of ship powered by purchased hydrogen could be higher. Thus, production of blue hydrogen onboard the ship offers a reasonably cost-effective low-carbon propulsion. However, the sub-systems have not been demonstrated onboard and so their development for a marine environment needs concerted effort. In addition, the good efficiency of the proposed system relies on an effective waste heat recovery system, which implies that retrofitting and redesigning ships need careful multi-partner collaborations including the manufacturer of the propulsion unit so that excessive back pressure is not imposed on it.
Concerning the advantages of this concept for meeting the IMO targets, the following comments can be made. The simplicity, flexibility, and scalability of the concept offer some advantages. The simplicity of PSA operation is an advantage of the proposed system. In terms of flexibility, the SMR-PSA concept is suitable for ships fitted with dual-fuel engines, irrespective of whether these are 2- or 4-stroke. One may also consider starting with a smaller degree of decarbonization in the beginning and reform and capture a higher percentage as time evolves. At this moment when the carbon taxes are much lower than the estimated CAPEX and OPEX of most decarbonization technologies, a full-scale investment on decarbonization technologies is an expensive solution. The system can be upscaled to meet the reduction targets from time to time. Moreover, the replacement of traditional diesel generators with fuel cells can also be considered for further improvement on ship energy and reduction efficiencies, However, the reformate may need further purification depending on the type of fuel cell employed. Finally, we should mention that the current proposal offers flexibility for future propulsion systems, e.g. based on hybrid modes, gas turbines, or fuel cells. Each of these applications would need optimization of the integrated reformer-CCS-combustion system following the methodology presented in this paper and an increase in TRL by pilot and demonstration projects before commercial deployment.
5 Conclusions
In this paper, an integrated reformer system with waste heat recovery was assessed thermodynamically. A comparison of SMR, ATR, and MPR hydrogen production units showed that SMR is the most energy-efficient hydrogen production system (εr = 71.5%), approximately 20% more energy efficient than ATR and MPR. SMR also has the highest hydrogen production rate, about 2% and 31% higher than ATR and MPR respectively. A comparison of CCS options (Table 4 part A) showed that PSA consumed 2.3 times, 5.3 times, and 6.8 times less energy than MEM, AA, and CS respectively in pre-combustion capture per unit mass of CO2. Hence, the SMR-PSA system was selected for further analysis. Optimization of the integrated system suggested to operate the reformer at 23.68%, 3.163, and 1072 °C for the operational parameters XCH4, S/C, and TSMR respectively. This operation yielded an optimal εo and βo of 41.7% and 64.9% respectively. From Fig. 8, it was also shown that a higher CO2 reduction rate was traded off with lower energy efficiency. This system was able to meet the IMO2030 CO2 reduction target (βo = 40%) with mixed fuel of 62% CH4 fed into the SMR-WGS-CCS-integrated system (Xf,SMR) and the remaining 38% of methane directly fed into the combustion engine. Full reforming reached 65% CO2 capture at an overall efficiency of 41%. This is not far from IMO2050, considering that the baseline for the IMO reduction is 2008 emissions with HFO operation. The efficiency penalty of the pre-combustion CCS/LNG reformer depends on the engine exhaust temperature (Fig. 9), implying that in practice the combination reformer/CCS/engine must be optimized together. The present results suggest that producing hydrogen onboard the ship and capturing the CO2 at the reformer stage offer an attractive marine decarbonization strategy.
Abbreviations
- AA:
-
Amine absorption
- ACAPEX:
-
Annualized capital cost
- ANOVA:
-
Analysis of variance
- ATR:
-
Autothermal reformer
- CH4 :
-
Methane
- CII:
-
Carbon intensity indicator
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- CS:
-
Cryogenic separation
- CT :
-
Total production cost of hydrogen
- EEDI:
-
Energy efficiency design index
- H:
-
Enthalpy
- Hfeed :
-
Enthalpy of feed
- HICE,feed :
-
Enthalpy of feed into ICE
- H2 :
-
Hydrogen
- H2O:
-
Water
- HFO:
-
Heavy fuel oil
- HTWGS:
-
High-temperature water-gas shift
- ICE:
-
Internal combustion engine
- IMO:
-
International Maritime Organization
- IMO2030:
-
IMO decarbonization goal of 40% CO2 reduction by 2030
- IMO2050:
-
IMO decarbonization goal of 70% CO2 reduction by 2050
- LNG:
-
Liquefied natural gas
- LTWGS:
-
Low-temperature water-gas shift
- mH2 :
-
Mass of produced hydrogen
- mH2,out :
-
Mass flow of hydrogen at reactor outlet
- MDEA:
-
Methyl diethanolamine
- MPR:
-
Methane pyrolysis reactor
- CCS:
-
Carbon capture and storage
- OCCS:
-
Onboard carbon capture and storage
- OPEX:
-
Operating cost
- PostCCS:
-
Post-combustion CCS
- PreCCS:
-
Pre-combustion carbon capture
- P:
-
Power
- PASU :
-
Power requirement of air separation unit
- PCCS :
-
Power requirement for CCS
- Pcomp :
-
Power requirement of methane compressor
- PH2O,pump :
-
Power requirement of water pump
- Pprop :
-
Propulsion power
- PSA:
-
Pressure swig adsorption
- PSMR :
-
Pressure of SMR
- Q:
-
Heat
- QCCS :
-
Heat requirement for CCS
- Qh,CH4 :
-
Heat produced by methane combustion in furnace
- Qh,SMR :
-
Heat requirement for SMR
- Qh,steam :
-
Heat requirement for steam generation
- S/C:
-
Steam to methane ratio
- SMR:
-
Steam methane reformer
- TRL:
-
Technological reliability level
- TSMR :
-
Exit temperature of SMR
- WGS:
-
Water-gas shift
- WHR:
-
Waste heat recovery
- WTW:
-
Well-to-wake
- XCH4 :
-
% feed of methane into furnace
- Xf,SMR :
-
% feed of methane into SMR-integrated system
- εo :
-
Energy efficiency
- εr :
-
Reactor efficiency
- βo :
-
CO2 reduction efficiency
- αH2 :
-
hydrogen yield
References
Mission Innovation, "Zero-emission shipping," Mission Innovation, 2022. http://mission-innovation.net/missions/shipping/. [Accessed 2 December 2022].
Christos Chryssakis, Pewe, H.: LNG as ship fuel. DNVGL (2021)
S. U. Khalid, "Safety features on LNG powered ships," Marine Insights, 28 June 2022. . https://www.marineinsight.com/marine-safety/safety-features-on-lng-ships/. [Accessed 06 December 2022].
Law, L.C., Mastorakos, E., Evans, S.: Estimates of the decarbonisation potential of alternative fuels for shipping as a function of vessel type, cargo, and voyage. Energies. 15(7468), (2022). https://doi.org/10.3390/en15207468
Law, L.C., Foscoli, B., Mastorakos, E., Evans, S.: A comparison of alternative fuels for shipping in terms of lifecycle energy and cost. Energies. 14(24), (2021). https://doi.org/10.3390/en14248502
Executive, T.M.: Concept to produce hydrogen on LNG ships to overcome obstacles. The Maritime Executive (2021) https://www.maritime-executive.com/article/concept-to-produce-hydrogen-on-lng-ships-to-overcome-obstacles. [Accessed 20 MArch 2023]
HyShip, "HyShip," HyShip, 2021. https://hyship.eu/. [Accessed 2 December 2022].
Wilhelmsen, "Wilhelmsen," Wilhelmsen, 17 December 2020.. https://www.wilhelmsen.com/media-news-and-events/press-releases/2020/wilhelmsens-topeka-hydrogen-project-awarded-nok-219-million/. [Accessed 2 December 2022].
A. BIOGRADLIJA, "HySeas III project to build hydrogen-powered ferry," Energy News, 7 June 2021. . https://energynews.biz/hyseas-iii-project-to-build-hydrogen-powered-ferry/. [Accessed 15 April 2022].
Imamura, T., Mogi, T., Wada, Y.: Control of the ignition possibility of hydrogen by electrostatic discharge at a ventilation duct outlet. Int. J. Hydrog. Energy. 34, 2815–2823 (2009). https://doi.org/10.1016/j.ijhydene.2009.01.028
Smith, J.R., Gkantonas, S., Mastorakos, E.: Modelling of boil-off and sloshing relevant to future liquid hydrogen carriers. Energies. 15, (2022). https://doi.org/10.3390/en15062046
IEA: The future of hydrogen seizing today’s opportunities- report prepared by the IEA for the G20, Japan. International Energy Agency, US (2019)
RINA: A scalable and sustainable proposal with hydrogen as fuel to meet IMO2050 targets. RINA https://www.rina.org/en/media/press/2021/11/25/ship-hydrogen-fuel. [Accessed 2 December 2022]
Marine Link: New ship technology will produce hydrogen on board from LNG. Marine Link https://www.marinelink.com/news/new-ship-technology-produce-hydrogen-501251. [Accessed 2 December 2022]
C-ZERO: Technology. C-ZERO (2021) https://www.czero.energy/technology. [Accessed 3 December 2022]
Kalamaras, C., Efstathiou, A.M.: Hydrogen production technologies: current state and future developmentS. Conf. Papers Energy. 2013, (2013). https://doi.org/10.1155/2013/690627
Steinberg, M.: Fossil fuel decarbonisation technology for mitigating global warming. Int. J. Hydrog. Energy. 24, 771–777 (1999)
Sánchez-Bastardo, N., Schlögl, R., Ruland, H.: Methane pyrolysis for zero-emission hydrogen production: a potential bridge technology from fossil fuels to a renewable and sustainable hydrogen economy. Ind. Eng. Chem. Res. 60, 11855–11881 (2021). https://doi.org/10.1021/acs.iecr.1c01679
Oni, A., Anaya, K., Giwa, T., Lullo, G.D., Kumar, A.: Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions. Energ. Conver. Manage. 254, (2022). https://doi.org/10.1016/j.enconman.2022.115245
Pashchenko, D.: Performance evaluation of a combined power generation system integrated with thermochemical exhaust heat recuperation based on steam methane reforming. Int. J. Hydrog. Energy. 48(15), 5823–5835 (2022). https://doi.org/10.1016/j.ijhydene.2022.11.186
Huang, Y., Zhang, Z., Long, Y., Zhang, Y., Li, G., Zhou, M.: Hydrogen production and energy efficiency optimization of exhaust reformer for marine NG engines: a view of surface reaction kinetics. Fuel. 336, (2023). https://doi.org/10.1016/j.fuel.2022.127051
Pruvost, F., Cloete, S., Pozo, C.A.D., Zaabout, A.: Blue, green, and turquoise pathways for minimizing hydrogen production costs from steam methane reforming with CO2 capture. Energ. Conver. Manage. 274, (2022). https://doi.org/10.1016/j.enconman.2022.116458
Pashchenko, D., Mustafin, R., Karpilov, I.: Thermochemical recuperation by steam methane reforming as an efficient alternative to steam injection in the gas turbines. Energy. 258, (2022). https://doi.org/10.1016/j.energy.2022.124913
Long, Y., Li, G., Zhang, Z., Wei, W., Liang, J.: Hydrogen-rich gas generation via the exhaust gas-fuel reformer for the marine LNG engine. Int. J. Hydrog. Energy. 47, (2022). https://doi.org/10.1016/j.ijhydene.2022.02.188
Yin, Z., Cai, W., Zhang, Z., Deng, Z., Li, Z.: Effects of hydrogen-rich products from methanol steam reforming on the performance enhancement of a medium-speed marine engine. Energy. 256, (2022). https://doi.org/10.1016/j.energy.2022.124540
Zhang, Z., Wu, R., Feng, S., Long, Y., Li, G.: Numerical investigation of tubular exhaust reformer with thermochemical recuperation for LNG engine. Int. J. Heat Mass Transf. 146, (2020). https://doi.org/10.1016/j.ijheatmasstransfer.2019.118743
Song, C., Liu, Q., Ji, N., Kansha, Y., Tsutsumi, A.: Optimization of steam methane reforming coupled with pressure swing adsorption hydrogen production process by heat integration. Appl. Energy. 154, 392–401 (2015). https://doi.org/10.1016/j.apenergy.2015.05.038
Kobayashi, A., Steinberg, M.: The thermal decomposition of Metane in a tubular reactor. Department of Applied Science - Brookhaven National Laboratory, New York (1992)
Ryi, S.-K., Park, J.-S., Kim, D.-K., Kim, T.-H., Kim, S.-H.: Methane steam reforming with a novel catalytic nickel membrane for effective hydrogen production. J. Membr. Sci. 339, 189–194 (2009). https://doi.org/10.1016/j.memsci.2009.04.047
Groote, A.M.D., Froment, G.F.: Simulation of the catalytic partial oxidation of methane to synthesis gas. Appl. Catal. Gen. 138, 245–264 (1996)
Law, L.C., Azudin, N.Y., Shukor, S.R.A.: Optimisation of operational parameter and economic analysis of amine based acid gas capture unit. Chem. Eng. Trans. 56, (2017). https://doi.org/10.3303/CET1756013
Zhao, Y., Zhao, D., Kong, C., Zhou, F., Jiang, T., Chen, L.: Design of thin and tubular MOFs-polymer mixed matrix membranes for highly selective separation of H2 and CO2. Sep. Purif. Technol. 220, 195–205 (2019). https://doi.org/10.1016/j.seppur.2019.03.037
Guido, G.D., Lange, S., Pellegrini, L.A.: Refrigeration cycles in low-temperature distillation processes for the purification of natural gas. J. Nat. Gas Sci. Eng. 27, 887–900 (2015). https://doi.org/10.1016/j.jngse.2015.09.041
Law, L.C., Othman, M.R., Mastorakos, E.: Numerical analyses on performance of low carbon containership. Energy Rep. 9, 3440–3457 (2023). https://doi.org/10.1016/j.egyr.2023.02.035
Stec, M., Tatarczuk, A., Iluk, T., Szul, M.: Reducing the energy efficiency design index for ships through a post-combustion carbon capture process. Int. J. Greenh. Gas Control. 108, (2021). https://doi.org/10.1016/j.ijggc.2021.103333
Feenstra, M., Monteiro, J., van den Akker, J.T., Abu-Zahra, M.R., Gilling, E., Goetheer, E.: Ship-based carbon capture onboard of diesel or LNG-fuelled ships. Int. J. Greenh. Gas Control. 85, 1–10 (2019)
Ji, C., Yuan, S., Huffman, M., El-Halwaji, M.M., Wang, Q.: Post-combustion carbon capture for tank to propeller via process modeling and simulation. J. CO2 Util. 51, (2021). https://doi.org/10.1016/j.jcou.2021.101655
GreenFacts, "CO2 capture and storage," GreenFacts, 5 April 2022. https://www.greenfacts.org/en/co2-capture-storage/l-3/4-transport-carbon-dioxide.htm. [Accessed 30 January 2023].
Hannu Jääskeläinen, "Natural gas engines," DieselNet, November 2022. https://dieselnet.com/tech/engine_natural-gas.php. [Accessed 31 December 2022].
NREL, “H2A-Lite: hydrogen analysis lite production model download,” https://www.nrel.gov/hydrogen/h2a-lite-download.html. [Accessed 21 May 2023].
Global Energy, Global Energy, 1 April 2022. https://globalenergyprize.org/en/2022/04/01/hydrogen-at-one-dollar-per-kilogram/. [Accessed 24 May 2023].
Lee, S., Kim, H.S., Park, J., Kang, B.M., Lim, H., Won, W.: Scenario-based techno-economic analysis of steam methane reforming process for hydrogen production. Appl. Sci. 11, (2021). https://doi.org/10.3390/app11136021
Honeywell: Honeywell Technology Enables Large U.S. Carbon Capture And Storage Project. Honeywell https://www.honeywell.com/us/en/press/2021/04/honeywell-technology-enables-large-us-carbon-capture-and-storage-project. [Accessed 22 March 2023]
Engineering, L.: Adsorption-based carbon capture and CO2 recovery. Linde Engineering https://www.linde-engineering.com/en/process-plants/adsorption-and-membrane-plants/adsorption-based-carbon-capture-and-co2-recovery/index.html. [Accessed 22 March 2023]
N. Simon, McCurdy, P.E. and H. M. Larson, “Examining the current and future economics of hydrogen energy,” https://www.icf.com/insights/energy/economics-hydrogen-energy. [Accessed 16 July 2023].
Kim, J.H., Park, D.K., Kin, T.J., Seo, J.K.: Thermal-structural characteristics of multi-layer vacuum-insulated pipe for the transfer of cryogenic liquid hydrogen. Metals. 12(4), (2022). https://doi.org/10.3390/met12040549
Funding
This research was funded by the National Research Foundation (NRF), Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.
Author information
Authors and Affiliations
Contributions
L.C.L., E.M., and A.T. were involved in conceptualization; L.C.L. and E.M. wrote the main manuscript and analysed the results; L.C.L. prepared all the tables and figures; and E.M., M.R.O., and A.T. reviewed and edited the article.
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Law, L.C., Mastorakos, E., Othman, M.R. et al. A Thermodynamics Model for the Assessment and Optimisation of Onboard Natural Gas Reforming and Carbon Capture. Emiss. Control Sci. Technol. 10, 52–69 (2024). https://doi.org/10.1007/s40825-023-00234-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40825-023-00234-z