Abstract
Objective
The aim of this study was to investigate the prevalence, severity, risk factors, and treatment outcomes of cyclophosphamide (CYC)-induced hemorrhagic cystitis (HC) in patients with rheumatic diseases.
Methods
We collected the clinical data from 1284 consecutive patients admitted to The First Affiliated Hospital of Sun Yat-Sen University who were treated with CYC between 2006 and 2016, and then conducted a retrospective analysis.
Results
The mean cumulative dose of CYC was 18.3 ± 13.4 g, and the mean treatment duration of CYC was 10.0 ± 7.2 months. We identified four patients with HC, yielding a crude prevalence of 0.3%. The average time from initial primary diagnosis to HC onset was 51.6 months (33–86 months). All of the four patients with HC were exposed to a high cumulative CYC dose (>60 g). Severity was assessed as grade II in one, grade III in one and grade IV in two patients. One had resolution of hematuria after hydration, and one case resolved after combination therapy of clot removal by cystoscopy, hydration, and bladder irrigation. The other two were unresponsive to the above treatment and finally had resolution after cystectomy. The average resolution time of hematuria was 39.5 days (7–56 days). There were no deaths in our cohort.
Conclusion
CYC-induced HC was rare and highly variable in Chinese patients with rheumatic diseases. Individualized treatment should be performed according to the severity of HC for each patient. More aggressive treatment strategies might improve the outcomes of patients with high-grade HC (grades III and IV). Our findings strengthened the link between HC events and higher cumulative CYC exposure (>60 g).
Similar content being viewed by others
Cyclophosphamide (CYC)-induced hemorrhagic cystitis (HC) was rare in Chinese patients with rheumatic diseases. |
HC was associated with higher cumulative CYC exposure (>60 g). |
1 Introduction
Cyclophosphamide (CYC), which is an oxazaphosphorine alkylating agent, has been widely used in the treatment of various malignancies as well as rheumatic diseases [1]. CYC is a cornerstone therapy for certain severe manifestations of rheumatic diseases, including lupus nephritis [2], scleroderma lung disease [3], and antineutrophil cytoplasmic antibody-associated vasculitis (AAV) [4]. Despite its effects, CYC is documented as having substantial adverse effects such as infections, bone marrow suppression, and urotoxicity. Hemorrhagic cystitis (HC) is a common manifestation of CYC-induced urotoxicity in rheumatic diseases [5], which varies between different countries and ethnicities [6,7,8]. However, few studies have addressed the prevalence of CYC-induced HC in Chinese patients with rheumatic diseases. Some observations indicated that the route of CYC administration and the cumulative doses could increase the risk of an HC event [6], while some argue to the contrary [7]. It has been unclear whether there is a cutoff dose that could predict the occurrence of HC. Moreover, there is considerable variability in the severity and treatment outcomes of patients with HC. The severity of HC could range from mild self-limiting hematuria to a potentially life-threatening presentation [9]. However, we are not aware of any study that provides any information about the clinical courses and outcomes of CYC-induced HC in rheumatic diseases.
In this study, we aimed to address the prevalence, clinical courses, as well as treatment outcomes of CYC-induced HC in rheumatism patients. We also attempted to evaluate the association between the usage of CYC (e.g., route of administration and the cumulative dose) and the occurrence of CYC-induced HC.
2 Materials and Methods
2.1 Patients and Study Design
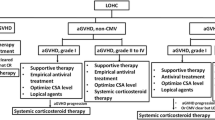
A retrospective study was performed to analyze the data of patients treated with CYC for severe manifestations of rheumatic diseases. All of the patients were consecutively admitted to The First Affiliated Hospital of Sun Yat-Sen University between 2006 and 2016. Either microscopic or macroscopic hematuria in the absence of urinary tract infection, nephrolithiasis, and erythrocyte casts was defined as HC. In all patients with hematuria, urinary tract ultrasonography was performed to exclude other causes of hematuria. Patients with abnormal ultrasonographic findings such as the presence of clots in the bladder were required to undergo cystoscopy. Cystoscopic confirmation was required for the final diagnosis of HC. Severity of HC was assessed based on the following criteria: Grade I—microscopic hematuria; Grade II—macroscopic hematuria; Grade III—macroscopic hematuria with small blood clots; Grade IV—severe hematuria with large blood clots, urinary obstruction, kidney damage, and bladder damage [10].
2.2 Cyclophosphamide Treatment
Intravenous (IV) CYC or daily oral CYC was prescribed based on the discretion of the physicians. As a general rule, IV CYC was administered every month at a dosage of 0.75 g/m2, or was prescribed at doses of 0.5 g/m2 in 1- to 2-week intervals. Daily oral CYC started at a dosage of 1 mg/kg per day and was increased to 2 mg/kg per day after 2 weeks provided that the leukocyte counts were >3.0×109/L. After remission, the regimens of CYC and alternative therapies were determined by the chief physician.
2.3 Data Collection
Demographic characteristics, the route of drug administration, cumulative dose of CYC, time span of CYC therapy, HC diagnosis, clinical courses as well as treatment outcomes were extracted from the medical records and analyzed.
2.4 Statistical Analysis
Computations were performed with SPSS 23.0. Continuous variables were expressed as the mean ± SD and compared using the t test. A chi-square test was conducted to compare the count data between the two groups. p values <0.05 (2-tailed) were considered significant.
3 Results
3.1 Basic Features of Enrolled Patients
The characteristics of the included 1284 patients with rheumatic diseases (952 female and 332 male) are summarized in Table 1. The mean age for all cases was 34.5 ± 3.9 years. Among 876 systemic lupus erythematosus (SLE) patients (731 women and 145 men, 31.4 ± 12.1 years of age), the underlying complications were lupus nephritis (528), neuropsychiatric lupus (101), pulmonary involvement (92), hematologic involvement (78), multi-system involvement (56), and antiphospholipid syndrome (11). AAV patients (193 cases; 107 women and 86 men, 37.4 ± 12.6 years of age) included 138 cases of granulomatosis with polyangiitis (GPA), 34 of eosinophilic granulomatosis with polyangiitis (EGPA), and 21 of microscopic polyangiitis (MPA). Seventy-two patients had Behçet’s disease (BD) with vascular involvement and 26 had neuro-Behçet. In the 117 patients with systemic sclerosis (SSc) (87 women and 30 men, 48.3 ± 14.2 years of age), 27 patients had limited SSc and 90 had diffuse SSc.
3.2 Administration of CYC and Cumulative CYC Dose
The mean cumulative doe of CYC was 18.3 ± 13.4 g, and the mean treatment duration of CYC was 10.0 ± 7.2 months. CYC was administered only intravenously in 1044 patients (81.3%), exclusively orally in 116 patients (9.0%), and by both routes in 124 patients (9.7%). In total, there were 240 patients (18.7%) ever treated with oral CYC. The mean cumulative dose of CYC and the treatment duration in the ever-oral group was significantly higher than that in the IV-only route (CYC dose: 40.4 ± 20.2 vs 14.6 ± 6.7 g; treatment duration: 20.7 ± 11.7 vs 8.2 ± 3.8 months, p < 0.001, respectively).
3.3 Hemorrhagic Cystitis (HC) Events
During the treatment of CYC, nonglomerular hematuria was detected in 26 patients. Of these, 18 were urinary tract infection, four were nephrolithiasis, one was bladder cancer, and four were HC. All of the four HC diagnoses were confirmed by cystoscopy, yielding a crude prevalence of 0.3%. Removable hyperechoic masses with no blood flow signal was the predominate feature detected by the ultrasound examinations (Fig. 1). Cystoscopy revealed prominent vascularity and submucosal petechial hemorrhages (Fig. 2). The detailed characteristics of the four patients with CYC-induced HC are listed in Table 2. The HC patients ranged in age from 41 to 75 years; all of them were female. HC began an average of 51.5 months after CYC treatment (range 33–86 months). Cases 1 and 2 had self-ingested oral CYC after disease remission, while the other two cases required repeat treatment of CYC due to chronic relapsing course. The mean cumulative CYC doses were higher for patients diagnosed with HC than those without HC (90.5 ± 44.2 vs 18.0 ± 12.2 g, p < 0.001). All of the HC events occurred in patients with a cumulative dose of CYC >60 g, thus suggesting a strong relationship between CYC cumulative dose and HC.
3.4 Clinical Outcomes and Treatments of HC
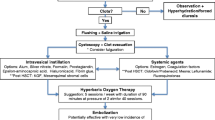
HC was grade II and III in one patient each, and grade IV in two patients. The interval between the initial hematuria and the withdrawal of CYC varied from 2 to 21 days. Case 4 with mild hematuria was treated with hydration only and hematuria resolved after 7 days. In case 3, after hydration and clot removal by cystoscopy, bladder irrigation with saline was performed. Hematuria resolved after 47 days of the above treatment. In cases 1 and 2, grade IV HC rapidly progressed, with urinary obstruction and hemorrhagic anemia, which needed blood transfusion. The two patients were unresponsive to less invasive treatments including hydration, continuous bladder irrigation, clot removal, and cauterization of bleeding sites by cystoscopy as well as interventional therapy of bilateral bladder artery embolization. With the suggestion of urologists, cystectomy with ileal conduit for bladder perforation was then performed in both these cases. Pathological diagnosis after operation showed that the bladders were characterized by marked edema and extensive hemorrhage throughout the lamina propria, telangiectasia, and inflammatory cell infiltration in the bladder walls. Blood clots were also found (Fig. 3). The two cases finally resolved after cystectomy. The average time to hematuria resolution was 39.5 days (7–56 days). Several risk factors were noted for patients to develop higher HC grade, including older age at HC onset and longer use of CYC after initial hematuria.
4 Discussion
This was a descriptive study of CYC-induced HC in rheumatic disease patients from China. Several key characteristics of CYC-induced HC were noted in the Chinese patients with rheumatic diseases. First, the prevalence of CYC-induced HC was exceptionally lower than that described in Western countries. Second, the severity of CYC-induced HC varied. Third, CYC-induced HC was prone to occur in patients with a high cumulative dose of CYC (>60 g).
CYC is considered to be the ‘strongest’ medication commonly used by rheumatologists in clinical practice to treat severe manifestations of systemic inflammatory diseases. CYC is widely used in China due to its cost effectiveness. Despite its efficacy, CYC is documented as having several life-threatening adverse effects including HC. Our results implied that the prevalence of CYC-induced HC (0.3%) was quite low in Chinese rheumatic patients. Another three cohorts from Asia (China, Hong Kong, and Korea) also reported that no HC events occurred during CYC treatment [11,12,13]. However, most European and American studies demonstrated a much higher prevalence of CYC-induced HC (1.5% in Turkey [7], 2.7% in France [6], 11.6% in Germany [14], and 82.4% in the US [15]) (Table 3). We diagnosed all cases of HC by cystoscopy in the current study, which indicated an accurate incidence.
Because the number of patients, the route of administration, and the cumulative doses were comparable to the studies from non-Asian populations [7], we inferred that the varied prevalence might be associated with ethnic difference. CYC is a pro-drug that undergoes complex metabolic activation and inactivation reactions, which are metabolized by cytochrome P450 (CYP), glutathione S-transferase (GST), and aldehyde dehydrogenase (ALDH) enzymes [16, 17]. Polymorphisms of these enzymes might affect its toxicity. The varied rate of CYC-induced HC between Asian and Western patients might be explained by the ethnic differences of gene polymorphisms of metabolic enzymes. A study from China indicated that GSTM1 and GSTT1 null mutations did not significantly alter the risk of side effects of pulsed high-dose CYC therapy in SLE patients [18]. There might be another mechanism. Ekhart et al. conducted a study in 113 Caucasian patients with solid tumors, and they discovered that patients that were heterozygous for the ALDH3A1*2 allele had an increased risk of HC when compared with patients with wild-type alleles (5/38 vs 1/70; p = 0.04) [19]. However, we demonstrated that Chinese SLE patients were not found to be heterozygous for the ALDH3A1*2 allele in our previous report [20]. The underlying mechanism needs to be further investigated.
The severity of HC in our cohort varied, which was similar to that reported in other studies. In a study by Kloos et al., severity was assessed as grade I in 7.1%, grade II in 57.1%, and grade III in 21.4% of the pediatric transplantation patients with CYC-induced HC [21]. A study from 1990 to 2010 in St. Jude Children’s Research Hospital (TN, USA) also reported a highly variable clinical course of CYC-induced HC [9]. Additionally, our study strengthened the link between higher HC grade and older age as well as longer use of CYC after hematuria. Further studies are needed to explore the severity of CYC-induced HC in rheumatic diseases.
Because the clinical course of HC is variable, individualized treatment should be adopted for each patient. For patients with low grade (I and II) HC, withdrawal of CYC and hydration may be effective. We used more aggressive treatment strategies than Gorczynska et al. and Hale et al. described in patients with high grade (III and IV) HC [22, 23]. The first step was clot evaluation and cauterization of actively hemorrhagic mucosa by cystoscopy. Next, patients should be treated with hydration, bladder catheter, and bladder irrigation. If patients fail to respond to the treatment, operations such as bilateral bladder artery embolization and cystectomy should be performed as a third-line option. Kaplan et al. also suggested additional cystoscopy was ineffective in those with CYC-induced HC [24], so alternate interventions such as operations should be considered if hematuria does not resolve after initial cystoscopy. There were no deaths in patients with high-grade HC in our center.
Because of the small numbers of cases of HC, multivariate analyses could not be performed. We could not conclude a confirmed association between gender and CYC-induced HC in our study. Another large cohort reported that gender was not a predictor for HC development [6].
Evidence for the effectiveness of mesna in preventing CYC-induced HC is uncertain in patients with rheumatic diseases. There were two studies suggesting that mesna might reduce the incidence rate of HC during oral CYC therapy [25, 26]. However, Yilmaz et al. observed a similar incidence rate for HC in both patient groups treated with or without mesna through an oral or IV route [7]. Mesna was not used in our clinical practice, and no IV CYC-induced HC was observed. Our study suggests that mesna may not be superior prophylaxis to our oral hydration protocol in preventing IV CYC-induced HC. Therefore, we speculate that patients with IV CYC might not benefit from prophylactic mesna administration. Prophylaxis with mesna should be recommended in patients with a high total cumulative dose of CYC, particularly with oral administration.
All of the four cases of CYC-induced HC had a high cumulative CYC dose (>60 g). Yilmaz et al. recently published a review on HC with CYC therapy in patients with mixed rheumatic diseases [7]. They found that cumulative CYC dose was the only significant factor associated with HC. A study from a French vasculitis study group also found that the urotoxicity risk in AAV was associated with the cumulative CYC dose [6]. Unlike the previous studies, we could not find an association between oral CYC administration and an HC event [6, 27]. However, older patients or patients with relatively less lethal situations when they are admitted to hospital may prefer oral CYC, and the poor medication compliance of some rural patients with transportation difficulties (the two cases of SSc in our study), which is a common situation in China, could possibly lead to high cumulative doses of CYC. Tailored treatment and recommendations should be carefully considered for these cases. Because of the small number of cases of HC in our study, we could not specify a cutoff point for cumulative CYC dose linked with increased HC risk. We suggest that clinicians need to pay close attention to HC events in patients with higher cumulative dose (>60 g), especially in older patients. Considering the expenses, and the very low prevalence of HC, we would not recommend intensive surveillance of urinary screening in CYC-treated patients, but for those at high risk and especially those who do not have good access to medical resources, alternative therapies should be considered for maintenance treatment when diseases are in remission. From another angle, CYC is quite safe with regard to HC in the Chinese population if cumulative dose is not extremely high.
This study had a few limitations. First, only patients followed up in the rheumatology clinic were enrolled, the subjects in the general family medicine clinic were missed, which might have led to selection bias. Second, because our study was a single-center study, it was impossible to overcome the bias caused by case source and the doctor’s medical practice. Third, because this study was a real-world study, some patients were inevitably lost to follow-up.
5 Conclusion
In conclusion, our data suggests that CYC-induced HC is rare and variable in Chinese patients with rheumatic diseases. Individualized treatment should be given according to severity for each patient. More aggressive treatment strategies might improve the outcomes of patients with high-grade HC. Our findings strengthened the link between HC events and higher cumulative CYC exposure (>60 g). Sustained awareness is needed for high-risk populations when prescribing this drug. The results of this retrospective study should be confirmed in larger prospective studies.
References
Brummaier T, Pohanka E, Studnicka-Benke A, et al. Using cyclophosphamide in inflammatory rheumatic diseases. Eur J Intern Med. 2013;24(7):590–6.
Karassa FB. Mycophenolate mofetil or intravenous cyclophosphamide in lupus nephritis. N Engl J Med. 2006;354(7):764–5.
Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66.
Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009;68(3):310–7.
de Jonge ME, Huitema AD, Rodenhuis S, et al. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005;44(11):1135–64.
Le Guenno G, Mahr A, Pagnoux C, et al. Incidence and predictors of urotoxic adverse events in cyclophosphamide-treated patients with systemic necrotizing vasculitides. Arthritis Rheum. 2011;63(5):1435–45.
Yilmaz N, Emmungil H, Gucenmez S, et al. Incidence of cyclophosphamide-induced urotoxicity and protective effect of mesna in rheumatic diseases. J Rheumatol. 2015;42(9):1661–6.
Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med. 1996;124(5):477–84.
Johnston D, Schurtz E, Tourville E, et al. Risk factors associated with severity and outcomes in pediatric patients with hemorrhagic cystitis. J Urol. 2016;195(4 Pt 2):1312–7.
Droller MJ, Saral R, Santos G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology. 1982;20(3):256–8.
Li J, Dai G, Zhang Z. General adverse response to cyclophosphamide in Chinese patients with systemic autoimmune diseases in recent decade-a single-center retrospective study. Clin Rheumatol. 2015;34(2):273–8.
Chan TM, Li FK, Hao WK, et al. Treatment of membranous lupus nephritis with nephrotic syndrome by sequential immunosuppression. Lupus. 1999;8(7):545–51.
Koo HS, Kim YC, Lee SW, et al. The effects of cyclophosphamide and mycophenolate on end-stage renal disease and death of lupus nephritis. Lupus. 2011;20(13):1442–9.
Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum. 2000;43(5):1021–32.
Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med. 1996;124(5):477–84.
Takada K, Arefayene M, Desta Z, et al. Cytochrome P450 pharmacogenetics as a predictor of toxicity and clinical response to pulse cyclophosphamide in lupus nephritis. Arthritis Rheum. 2004;50(7):2202–10.
Ekhart C, Doodeman VD, Rodenhuis S, et al. Influence of polymorphisms of drug metabolizing enzymes (CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, GSTA1, GSTP1, ALDH1A1 and ALDH3A1) on the pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide. Pharmacogenet Genomics. 2008;18(6):515–23.
Zhong S, Huang M, Yang X, et al. Relationship of glutathione S-transferase genotypes with side-effects of pulsed cyclophosphamide therapy in patients with systemic lupus erythematosus. Br J Clin Pharmacol. 2006;62(4):457–72.
Ekhart C, Rodenhuis S, Smits PH, et al. Relations between polymorphisms in drug-metabolising enzymes and toxicity of chemotherapy with cyclophosphamide, thiotepa and carboplatin. Pharmacogenet Genomics. 2008;18(11):1009–15.
Lingyan C, Xueding W, Li Jiali, et al. Relationship between aldehyde dehydrogenase polymorphisms and cyclophosphamide-induced adverse effects in patients with systemic lupus erythematosus. Chin J Clin Pharmacol. 2014;03:163–6.
Kloos RQ, Boelens JJ, de Jong TP, et al. Hemorrhagic cystitis in a cohort of pediatric transplantations: incidence, treatment, outcome, and risk factors[J]. Biol Blood Marrow Transplant. 2013;19(8):1263–6.
Gorczynska E, Turkiewicz D, Rybka K, et al. Incidence, clinical outcome, and management of virus-induced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11(10):797–804.
Hale GA, Rochester RJ, Heslop HE, et al. Hemorrhagic cystitis after allogeneic bone marrow transplantation in children: clinical characteristics and outcome. Biol Blood Marrow Transplant. 2003;9(11):698–705.
Kaplan JR, Wolf JJ. Efficacy and survival associated with cystoscopy and clot evacuation for radiation or cyclophosphamide induced hemorrhagic cystitis. J Urol. 2009;181(2):641–6.
Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum. 2000;43(5):1021–32.
Gourley MF, Austin HR, Scott D, et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125(7):549–57.
Monach PA, Arnold LM, Merkel PA. Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: a data-driven review. Arthritis Rheum. 2010;62(1):9–21.
Acknowledgements
The project was supported by the National Natural Science Foundation of China (No. 81102270, 81603435), Guangdong Natural Science Foundation (No. 2014A030313053, No. 2016A030313217), and Guangdong Technology Project (No. 2014A020221009, No. 2014A020212119, No. 2016A020215051).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors (Liuqin Liang, Donging Chen, Xiaodong Wang, Zheng Yang, Jun Zhou, Zhongping Zhan, Fan Lian) declare that they have no conflicts of interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liang, L., Chen, D., Wang, X. et al. Rare Cyclophosphamide-Induced Hemorrhagic Cystitis in a Chinese Population with Rheumatic Diseases. Drugs - Real World Outcomes 4, 175–182 (2017). https://doi.org/10.1007/s40801-017-0112-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40801-017-0112-y