Abstract
Purpose of Review
This review aims to provide an update on the recent major advances in the management of retinopathy of prematurity (ROP).
Recent Findings
There have been a number of major advances in our understanding and management of ROP over the last decade: (1) The advent of improved imaging techniques and technological infrastructure has led to the increased use of telemedicine and potential use of artificial intelligence to enhance access to care for children at risk of developing ROP; (2) the International Classification of Retinopathy of Prematurity (ICROP) 3rd edition has provided updates in classification of ROP and response of ROP to treatment; and (3) the treatment paradigm has shifted from laser therapy exclusively to now having the option of anti-vascular endothelial growth factor (VEGF) therapy. This has led to greater interest in trying to better understand the possible adverse events related to systemic and local VEGF suppression.
Summary
There is a greater understanding in the diagnosis and treatment of ROP and its response to treatment. The advent of anti-VEGF therapy has provided ROP providers with a treatment modality that may lead to improved visual outcomes without the need for peripheral retinal ablation. However, there remain questions regarding systemic and local adverse events. Laser photocoagulation continues to be an effective primary therapy and may also be needed after or in conjunction with anti-VEGF treatment.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative retinal disease of the developing retina and is one of the leading causes of visual morbidity and blindness in preterm infants worldwide [1]. Data from the National Eye Institute estimated that about 14,000–16,000 preterm infants in the USA annually have some degree of ROP. About 4001600 infants each year in the USA are legally blind from ROP. Historically, three epidemics have been described. From the 1940s to 1970s, the first and second epidemic of ROP occurred in high-income countries primarily due to unmonitored supplemental oxygen and higher survival rates of preterm babies with increased use of neonatal intensive care units (NICU), respectively [2]. Currently, the third epidemic, which originated in the 1990s in low- and middle-income countries (LMIC), is due to higher survival rates of preterm babies as a result of improved NICU access, improved neonatal survival, and other factors such as limited ROP awareness and screening services [3]. It has been estimated that the preterm infants at risk of ROP in these LMICs were 82% of the total worldwide in 2010 [4]. Moreover, ROP in these developing regions has also been found to develop in more mature and larger babies [5].
There have been many recent advances in the field of ROP including the use of novel imaging techniques, telemedicine and artificial intelligence to screen ROP, and a shift of the treatment paradigm from laser photocoagulation to intravitreal anti-VEGF therapy [6, 7, 8••]. More is needed to learn of how each advancement can be integrated into clinical practice in a practical, efficient, and cost-effective manner. This review aims to summarize the current state of ROP management in order to provide an updated evidence-based approach to this blinding pediatric retina condition.
Pathogenesis of ROP
The pathogenesis of ROP has been described to be of two phases [9•]. In phase 1, there is delay in physiologic retinal vascular development in preterm birth and a hyperoxia-induced vasco-attenuation [9]. This is followed by phase 2 where there is vaso-proliferation into the vitreous cavity. An important molecule involved in this process in vascular endothelial growth factor (VEGF) [10]. Non-perfused (i.e., ischemic) retina results in an upregulation of VEGF, which is an angiogenic factor that causes neovascularization. If unchecked, this can result in fibrovascular tissue formation and retinal detachment [11]. It is important, however, to note that normal levels of VEGF are required for systemic development of the blood-brain barrier, kidneys, lungs, and skeletal and hematopoietic systems [10].
Classification of ROP
The classification of acute ROP was developed by the International Classification of Retinopathy of Prematurity (ICROP) group first in 1984, then expanded in 1987 [12]. This was based on clinical examination which classified acute ROP into five stages, three zones, and extent of retinal involvement. An additional feature included the presence of dilated and tortuous posterior pole vessels known as “plus” disease. An entity known as aggressive posterior ROP (APROP) was defined in 2005 to describe a posterior disease with prominent plus and ill-defined retinopathy that may rapidly progress to stage 5 disease [12]. Pre-plus disease was also described to include eyes with vessel tortuosity that did not meet “plus” criteria. Over the years, progress has been made in ophthalmic imaging and treatment options. Increasingly, we are aware of global differences in terms of ROP patterns, as well as subjectivity among ROP experts [8]. This provided the impetus for the ICROP 3rd edition (ICROP3) which provides further guidance [8]. Important additions in ICROP3 include refined classification metrics such as “posterior zone II,” a notch, subcategorization of stage 5, recognition of a spectrum of vascular abnormality defining plus disease, the use of the term aggressive ROP (AROP) instead of specifically APROP, and description of regression, reactivation, and long-term sequelae.
Screening for ROP
Due to global variation on ROP phenotypes, screening criteria should be tailored to the individual region. In the USA, for example, the recommendation for screening includes a birth weight of less than or equal to 1500 g or gestational age of 30 weeks or less. Infants of birth weight between 1500 and 2000 g or gestational age of more than 30 weeks should also be screened if the clinical course is unstable, including those requiring cardiorespiratory support or determined as high risk by the neonatologist [13]. In LMICs, there are regional differences that may not allow for a universal guideline for screening [14]. Moreover, applying screening guidelines from developed countries to LMICs may fail to identify larger and more mature infants who develop ROP [15, 16].
Telemedicine and Artificial Intelligence for ROP
With the rise of the third epidemic of ROP, there is an increasing need for widespread, affordable, and user-friendly screening for timely identification of at-risk infants. Telemedicine for ROP has been implemented in many areas of the world and artificial intelligence (AI) is currently under investigation as a means to improve access to care. Telemedicine in ophthalmology has been shown to successfully provide synchronous visits for low-income and uninsured patients in a tertiary university-based setting [17]. The framework for telemedicine programs for ROP offers solutions to many of the challenges of the third epidemic of ROP by means of nonphysician imagers and interpreters to grade ROP with accuracy to allow for timely management of cases requiring treatment [18, 19]. Telemedicine programs typically operate by means of trained nonphysician imagers to obtain accurate images to send to specialists who then confirm diagnosis and can facilitate referral for treatment in a timely manner [6•, 7•]. Some examples of successful ROP telemedicine screening programs include the Karnataka Internet-assisted Diagnosis of Retinopathy of Prematurity (KIDROP), the Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP), and Retinopathy of Prematurity Eradication-Save Our Sight (ROPE-SOS) [6•].
Telemedicine screening programs have also allowed for the development of training programs for nonphysicians and AI systems for ROP diagnosis by harvesting the digital images taken during telemedicine sessions to be used for education [20]. This has the potential to create a sustainable, affordable, and robust network of data and images to continue to expand ROP education and management.
Both telemedicine and AI seek not to replace the workforce, but rather to empower, expand, and unite it through user-friendly, validated, and reliable operation [6•]. Data from a study by Daniel et al. suggested that the training system used to certify nonphysicians to use telemedicine approaches to evaluating acute-phase ROP (e-ROP) revealed correct detection of serious ROP with good intra- and intergrader consistencies with significantly minimal temporal drift [21]. This not only allows for expansion of the workforce but also unified grading through lowering the variability that exists between examiners diagnosing ROP [22]. The Global Education Network for Retinopathy of Prematurity (GEN-ROP) initiated aims to improve ROP education among trainees [23]. An open-access online web-based platform was developed to teach, assess, and improve the diagnostic accuracy of ROP. Not only did it become a preferred pedagogical method, it was also found to significantly improve the diagnostic accuracy and reliability of ROP diagnosis among an international group of ophthalmology trainees [23, 24]. This timely effort was able to address the unmet need of limited ROP training among ophthalmology trainees which may result in misdiagnoses and mismanagement [25].
Campbell et al. demonstrated that diagnosis of ROP through the use of fundus imaging was similar in accuracy compared to indirect ophthalmoscopy [26]. The Imaging and Informatics in Retinopathy of Prematurity Deep Learning (i-ROP DL) system that had been granted breakthrough status by the US Food and Drug Administration was developed by the i-ROP consortium and it utilizes convolutional neural networks (CNNs) for vessel segmentation and assessment of plus disease [27]. Notably, the i-ROP DL system has been found to diagnose plus disease as good and even better than experts (92% vs. 82% accuracy); it has potential to predict progression to treatment requiring ROP; and can also be used to survey regression post treatment [28, 29, 30]. Specifically, the i-ROP system generates a vascular severity score (VSS) [1, 2, 3, 4, 5, 6, 7, 8, 9] for ROP [31•]. In addition, the CNN was found to be accurate in the diagnosis of ROP stages 1, 2, and 3 in fundus images from datasets in North America and Nepal [32]. When modeled, gestational age plus VSS from a single examination (at 32 to 33 weeks postmenstrual age) was found to identify all infants who developed treatment requiring ROP more than 1 month before diagnosis with moderate to high specificity [33].
Furthermore, in terms of generalizability, i-ROP DL was externally validated in an Indian ROP telemedicine program [34]. We found the receiver operating characteristic curve for detecting treatment requiring ROP was 0.98, with 100% sensitivity and 78% specificity. A higher ROP severity was also found in neonatal care units without oxygen blenders and pulse oxygenation monitors. This study showed that AI can be successfully applied into ROP screening programs and can potentially lead to better care and distribution of resources for neonatal care.
Management of ROP
Observation
Most cases of ROP resolve spontaneously. The landmark Cryotherapy for ROP (CRYO-ROP) trial showed that ROP resolves in 80% of cases and the Early Treatment for ROP (ETROP) trial showed that only 10% of infants meet criteria for ROP treatment [35, 36].
Cryotherapy
The first description of cryotherapy to ablate peripheral ischemic tissue in infants with ROP was as early as 1972 from Japan [37]. The CRYO-ROP group showed that treatment of threshold disease with cryotherapy reduced the rate of unfavorable outcomes by 50%, as defined by partial retinal detachment, cataracts, retrolental membrane, corneal opacity, or total retinal detachment [38]. The treatment effect persisted up until 15 years when it was found that 30% of treated eyes compared to 51.9% control eyes had unfavorable structural outcomes [35]. Threshold disease was defined as 5 or more clock hours of contiguous or 8 total clock hours of stage 3 ROP in zone I or II with plus disease.
Currently, although cryotherapy is rarely performed, physicians are managing the long-term effects of the disease and its treatment. Studies have shown that 4.5% of treated eyes continue to develop new retinal folds or retinal detachments after 10 years of age [35].
Laser Photocoagulation
The ETROP trial supported the use of laser photocoagulation to treat prethreshold disease [36]. Eyes with prethreshold disease were classified as type 1 ROP where treatment is indicated within a time frame of 48 to 72 h and type 2 ROP where close follow-up is indicated. Type 1 ROP was defined as 1) zone I, any stage with plus; 2) zone I, stage 3 without plus; and 3) zone II, stage 2 or 3 with plus. In the ETROP trial, treatment of type 1 ROP reduced unfavorable structural outcomes from 15.6 to 9.0% and unfavorable visual acuity outcomes from 19.8 to 14.3% [39]. Laser was advantageous to cryotherapy especially in the treatment of zone I disease. Moreover, cryotherapy had been shown to be associated with poor structural and functional outcomes in the treatment of zone I disease [40]. Lastly, it is important to note that skip areas following laser can lead to reactivation of disease [7].
Anti-vascular Endothelial Growth Factor (VEGF) Therapy
Over the past 15 years, there has been a shift to the use of intravitreal anti-VEGF for the treatment of ROP [41]. A Cochrane review in 2018 included five trials that compared bevacizumab or ranibizumab with conventional laser therapy and one that compared intravitreal pegaptanib plus laser vs. laser/cryotherapy. It was found that bevacizumab/ranibizumab monotherapy reduces the risk of refractive error during childhood but does not reduce the risk of retinal detachment or recurrence of ROP in infants with type 1 ROP [41]. While treatment reduces the risk of recurrence in zone I ROP, it may potentially result in a higher risk of recurrence in zone II ROP. The long-term systemic effects were also not known from this review. Hence, the authors concluded that the evidence supporting the use of anti-VEGF was not robust enough.
In general, the advantages of anti-VEGF therapy include the ease of performing the procedure, faster regression of ROP, lower risk of myopia, less stress on the infant, and faster administration time [42, 43]. Compared to laser, anti-VEGF is also useful for treating posterior disease and APROP.
The first published results of a prospective randomized clinical trial that compared bevacizumab with conventional laser were from the BEAT ROP trial [44]. The authors found that bevacizumab reduced the risk of reactivation before 54 weeks of postmenstrual age by five times compared to conventional laser therapy for infants with zone I disease. However, the trial did not look at mortality, local or systemic adverse events [45]. Subsequently, the RAINBOW study (ClinicalTrials.gov identifier NCT02375791) compared ranibizumab to laser therapy. At 24 weeks post treatment, success was achieved in 80% of infants with 0.2 mg ranibizumab, 75% with 0.1 mg ranibizumab, and 66.2% following laser treatment [46•]. At 2 years, no new structural abnormalities were reported and ranibizumab 0.2 mg was still superior to laser therapy and causes less myopia [47]. The 5-year extension trial of the RAINBOW study will provide more evidence on the long-term effects of ranibizumab for the treatment of ROP. Importantly, both the BEAT-ROP and RAINBOW studies have surprisingly low success rates following laser as compared to the earlier ETROP trials [39]. Another anti-VEGF agent, aflibercept, is currently being investigated prospectively for the treatment of ROP in the BUTTERFLEYE and the FIREFLEYE trials (ClinicalTrials.gov NCT04101721 and NCT 4004208).
There have been concerns about the potential systemic adverse effects associated with anti-VEGF therapy in infants, especially in terms of neurodevelopmental outcomes. Numerous investigators including those of the BEAT-ROP and RAINBOW trials have reported on this. Kaushal et al. performed a meta-analysis of thirteen studies (1 clinical trial and 12 cohort studies) and found that bevacizumab for severe ROP was associated with an increased risk of cognitive impairment (adjusted odd ratio 1.90; 95% CI 1.22, 2.97) and lower cognitive and language scores in preterm infants when compared to infants treated with laser or cryotherapy [48]. However, Tsai et al. found in their meta-analysis of eight studies that the risk of severe neurodevelopmental impairment was not increased in ROP infants after bevacizumab treatment [49•]. In the first prospective randomized controlled trial that looked at neurodevelopmental outcomes, Marlow et al. of the RAINBOW study group found that at 20–28 months corrected age there were similar neurodevelopmental scores in the ranibizumab 0.2 mg group vs. laser group [47].
In view of the systemic concerns, there has also been ongoing research to determine if lower doses of anti-VEGF can be given with similar success. The PEDIG group found that treatment success was 90% in 0.004 mg of bevacizumab and 74% for 0.002 mg of bevacizumab [50]. One potential issue with lower doses of anti-VEGF is the difficulty in drawing the correct volume in the clinic setting. For ranibizumab, the CARE-ROP group also found similar efficacy with both 0.12 mg and 0.2 mg. Moreover, the authors also found that peripheral normal vascularization developed more rapidly and occurred more frequently in the 0.12 mg group [51].
The indication for the use of anti-VEGF has also been expanded to include perioperative use. Xu et al. compared the use of pre-operative bevacizumab vs. without bevacizumab for vascularly active stage 4 ROP. The authors found that bevacizumab reduced active vascularisation prior to vitrectomy, resulted in a shorter surgical time, and had better anatomical and functional success [52]. However, this study was retrospective in nature. In another study, Kychenthal et al. also found that pre-operative bevacizumab (1 week prior to surgery) reduced vascular activity and was a feasible option. More studies are needed to clarify the role and efficacy of pre-operative anti-VEGF in ROP requiring surgery [53].
Surgery for Stage 4 and 5 ROP
When retinal detachment occurs in advanced ROP, it is classified as stage 4 or 5. Stage 4A is defined as partial retinal detachment with macula sparing, while stage 4B is defined as partial retinal detachment with involvement of the macula. The ICROP 3rd edition further classified stage 5 (total retinal detachment) into subcategories: stage 5A, in which the optic disc is visible by ophthalmoscopy (suggesting open-funnel detachment); stage 5B, in which the optic disc is not visible due to retrolental fibrovascular tissue or closed-funnel detachment; and stage 5C, in which stage 5B is accompanied by anterior segment changes (e.g., marked anterior chamber shallowing, irido-corneo-lenticular adhesions, corneal opacification), suggesting closed-funnel configuration [8].
The options of surgery include vitrectomy with or without scleral buckling and scleral buckle alone. Lensectomy can be performed to improve access to anterior structures but brings with it risk of refractive amblyopia and a potentially higher risk of glaucoma [54]. In a large series by Nudleman et al., lens sparing vitrectomy was performed for stages 4A, 4B ,and 5 ROP. Anatomical success was achieved in 82.1% for stage 4A, 69.5% for 4B, and 42.6% for stage 5 [55]. The risks of surgery include cataract, corneal opacity, glaucoma, and strabismus [56]. In general, the prognosis is poor for stage 5 ROP.
Follow-up of ROP
Following ROP screening, if no treatment is indicated, subsequent clinical examination will depend on the stage and zone of ROP. Follow-up examinations of less than 1-week, 1- to 2-week, and 2- to 3-week intervals have been suggested. In general, cessation of clinical examination can be based on age and clinical findings. The criteria include zone III retinal vascularization attained without previous zone I or zone II ROP, full retinal vascularization near the ora serrata for 360 degrees, postmenstrual age of 50 weeks, and no prethreshold disease, or regression of ROP [57].
Regression refers to disease involution and resolution, which can occur spontaneously or with treatment [8]. When there is persistence of retinal abnormalities, it is known as incomplete regression. Regression is usually more rapid following anti-VEGF treatment (1–3 days) as compared to laser photocoagulation (7–14 days). Clinical signs of regression include decreased “plus” disease, reduced vascular dilatation and tortuosity, involution of tunica vasculosa lentis, better pupillary dilation, improved media clarity, resolution of intra-retinal hemorrhage, and thinning and whitening of neovascular tissue. Subsequently, there will be normal vascularization of previously avascular peripheral retina. With the shift to increased use of anti-VEGF, new retinal abnormalities have been observed. Lepore et al. found that eyes treated with bevacizumab showed peripheral and posterior pole angiographic abnormalities such as large avascular areas, abnormal branching and shunting, hyperfluorescent lesions, and absence of foveal avascular zone [58, 59]. Incomplete vascularization is termed as persistent avascular retina (PAR), and currently there is no established consensus regarding treatment.
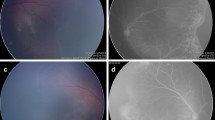
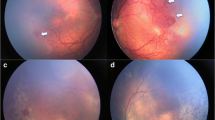
Reactivation of previously treated ROP varies from a minimal self-limiting demarcation line to stage 3 plus disease [8]. Following laser treatment, skip areas can contribute to reactivation [60]. However, reactivation usually occurs earlier (before 55 weeks post menstrual age) following laser treatment as compared to anti-VEGF (Fig. 1). The BEAT ROP study showed that mean time of reactivation was 16 weeks post-treatment with bevacizumab [44]. Late reactivation has been described up until 60 weeks post menstrual age [7].
Fundus images showing reactivation of ROP (post IVB* treatment) and regression with subsequent laser photocoagulation. A Pre-IVB at 35-week PMA^ showing zone II stage 3+ ROP. B Post-IVB at 36-week PMA showing regression of plus disease and reduced neovascularization. There is also progression of normal vascularization toward the periphery. C Early reactivation of ROP at 46 weeks (recurrence of plus disease and more posterior demarcation line). D Post rescue laser photocoagulation at 49 weeks. *Intravitreal bevacizumab; ^ post menstrual age.
In the long term, the sequelae of preterm infants with or without ROP can be classified into ocular and systemic. Even in preterm infants without ROP, subtle visual deficits can occur [61]. The ocular sequelae of resolved ROP include lattice-like degeneration, cicatricial changes, retinal folds, retinal detachments, or schisis [62]. There is also risk of myopia that is worse after laser photocoagulation as compared to anti-VEGF [47]. The risk of myopia is higher with treatment of posterior ROP with cryotherapy or laser [63, 64]. Other ocular complications include cataracts, angle closure glaucoma, strabismus, and reduced visual fields [40, 65, 66].
The preterm infant may also have systemic adverse effects associated with treatment after laser or anti-VEGF. Apnea and bradycardia have been reported with laser treatment [67]. Intravitreal administration of anti-VEGF can also result in serum VEGF suppression. This effect has been shown to be less pronounced with ranibizumab and aflibercept as compared to bevacizumab [68, 69]. As previously mentioned, it is still unclear if anti-VEGF may result in adverse neurodevelopmental outcomes, and more prospective studies are required in this area [47, 48, 49].
Novel imaging for ROP
Imaging for ROP, especially wide-field imaging systems, is useful for classification and to monitor progression and regression of disease. Digital imaging systems such as the RetCam (Natus Medical Systems, Inc., Pleasanton, CA, USA) have been commonly utilized and interpretation of retinal images has been shown to have high specificity and sensitivity for ROP diagnosis [70]. However, potential issues with resolution and cost are the main limitations in the routine use of digital imaging. New systems have emerged over the past decade. The ICON system (Phoenix Clinical, Inc., Pleasanton, CA) is a contact-based handheld mydriatic device that has advantages with dark fundi, such as improved color profile [71]. Similarly, the PanoCam LT widefield system (Visunex Medical System) is contact based, wireless, offering a field of 130° [72]. The camera attachment of the Heidelberg Spectralis ultra-wide field (Heidelberg Engineering) offers a non-contact system with a field of 102° and has been used for exams under anesthesia [72]. The 3nethra Neo (Forus Health) is a contact based, lower cost, and non-wireless system that offers a field of 120° [72]. It is a portable system with telemedicine integration that allows for use in multiple settings and conditions at a cost of around 1/5 of comparable systems [73].
Implementation of fluorescein angiography (FA) in imaging modalities, available in multiple systems such as the RetCam, ICON, Spectralis among others, is becoming an integral aspect of ROP monitoring and diagnosis, especially in the anti-VEGF era, as FA can identify vascular changes not evident during direct examination. Similarly, optical coherence tomography (OCT), which allows for the detection of the morphological features in ROP, is becoming more common in ROP screening and follow-up, and OCT and OCTA handheld systems have been developed [74, 75].
With the software and technological advances of smartphone camera systems, smartphone-based fundus imaging has become another low-cost and easily transportable avenue for imaging ROP patients. The Paxos Scope smartphone adapter (DigiSight Technologies Inc./Verana Health Inc., San Francisco, California) utilizes a smartphone and an indirect lens to provide fundus images. The system has been validated for its use in ROP screening utilizing an iPod touch (6th generation, Apple Inc., Cupertino, California) and a Pan Retinal 2.2 lens (Volk Optical Inc., Mentor, Ohio). Specifically, the sensitivity and specificity for detection of ROP and plus disease was comparable to contact-based fundus imaging systems. Limitations of smartphone-based adapters include longer examination times and a smaller field of view (56°) compared to contact-based systems [76]. Similarly, other low-cost smartphone adapters such as the RetinaScope and MII Ret Cam have been validated for ROP screening as well [77, 78].
Conclusions and Future Directions
Retinopathy of prematurity remains one of the leading causes of visual morbidity and blindness in infants worldwide. As healthcare systems continue to advance and knowledge of ROP continues to improve, the incidence of ROP will likely continue to increase in the coming decades. This review summarizes the current state of treatment and diagnosis of ROP as well as the current and future applications of imaging modalities, AI, and telemedicine to better manage infants with ROP. The key points of this review include the following.1) Classification systems continue to evolve with the ICROP 3rd edition. 2) A one-size-fits-all approach to ROP screening cannot be applied worldwide, and regional differences as well as the individual clinical course of the patient population need to be considered. 3) Telemedicine continues to expand and is becoming an integral part in the diagnosis and management of ROP worldwide. Artificial intelligence is currently being investigated for clinical care of patients with ROP. 4) Laser photocoagulation continues to be effective for treatment. Anti-VEGF is more available and has become the primary treatment for many clinicians. 5) Newer, more affordable imaging systems have been developed that allow for an expanded role for telemedicine and potentially AI in the management of ROP.
A number of questions remain unanswered regarding the management of ROP. Specifically, the long-term safety effects of anti-VEGF agents in infants, the correct dose to use, and the long-term outcomes of anti-VEGF treatment. Also, no consensus exists for the frequency of exams and length of follow-up after treatment with anti-VEGF as well as time to discharge, and whether to treat or not treat the persistent avascular retina. Long-term follow-up studies, including the 5-year extension of RAINBOW as well as BUTTERFLEYE and FIREFLEYE, will hopefully elucidate these questions. Finally, one of the major challenges to ROP treatment and screening in rural areas low- and middle-income countries includes access to trained physicians and imaging systems. With the development of low-cost, easily transportable imaging modalities with integration of telemedicine and AI platforms, diagnosis and access to care will become less of a barrier.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382(9902):1445–57. https://doi.org/10.1016/S0140-6736(13)60178-6.
Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77–82. https://doi.org/10.1016/j.earlhumdev.2007.11.009.
Darlow BA, Gilbert C. Retinopathy of prematurity - a world update. Semin Perinatol. 2019;43(6):315–6. https://doi.org/10.1053/j.semperi.2019.05.001.
Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2. https://doi.org/10.1186/1742-4755-10-S1-S2.
Quinn GE. Retinopathy of prematurity blindness worldwide: phenotypes in the third epidemic. Eye Brain. 2016;8:31–6. https://doi.org/10.2147/EB.S94436.eCollection2016.
Al-Khaled T, Valikodath NG, Patel SN, Cole E, Chervinko M, Douglas CE, et al. Addressing the third epidemic of retinopathy of prematurity through telemedicine and technology: a systematic review. J Pediatr Ophthalmol Strabismus. 2021;58(4):261–9. https://doi.org/10.3928/01913913-20210223-01This review showed that the use of telemedicine and technology is a viable solution for ROP screening especially in low income countries. It has been shown to be cost-effective but requires national support.
Tsai AS, Chou HD, Ling XC, Al-Khaled T, Valikodath N, Cole E, et al. Assessment and management of retinopathy of prematurity in the era of anti-vascular endothelial growth factor (VEGF). Prog Retin Eye Res. 2021:101018. https://doi.org/10.1016/j.preteyeres.2021.101018This review summarized the current state of ROP management, given the rising use of anti-VEGF. This paper also suggested future directions of research such as cost effective imaging devices, implementation of AI platforms and updated algorithms in the treatment of ROP.
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International classification of retinopathy of prematurity, Third Edition. Ophthalmology. 2021;128(10):e51–68. https://doi.org/10.1016/j.ophtha.2021.05.031This landmark paper provided the updated classification for ROP. Importantly, it formally recognized plus disease as being as spectrum of vascular abnormality. A new term AROP was also used to replace APROP.
Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122(1):200–10. https://doi.org/10.1016/j.ophtha.2014.07.050This paper discussed detailed pathomechanisms in ROP based on animal models. It highlighted the importance of VEGF receptor 2 signaling and the importance of inhibiting aberrrant angiogenesis while promoting physiologic retinal vascular development.
Chan-Ling T, Gole GA, Quinn GE, Adamson SJ, Darlow BA. Pathophysiology, screening and treatment of ROP: a multi-disciplinary perspective. Prog Retin Eye Res. 2018;62:77–119. https://doi.org/10.1016/j.preteyeres.2017.09.002.
Agarwal K, Jalali S. Classification of retinopathy of prematurity: from then till now. Commun Eye Health. 2018;31(101):S4–7.
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991–9. https://doi.org/10.1001/archopht.123.7.991.
Fierson WM, AMERICAN ACADEMY OF PEDIATRICS Section on Ophthalmology, AMERICAN ACADEMY OF OPHTHALMOLOGY, AMERICAN ASSOCIATION FOR PEDIATRIC OPHTHALMOLOGY AND STRABISMUS, AMERICAN ASSOCIATION OF CERTIFIED ORTHOPTISTS. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142(6). https://doi.org/10.1542/peds.2018-3061.
Mora JS, Waite C, Gilbert CE, Breidenstein B, Sloper JJ. A worldwide survey of retinopathy of prematurity screening. Br J Ophthalmol. 2018;102(1):9–13. https://doi.org/10.1136/bjophthalmol-2017-310709.
Chen Y, Feng J, Li F, Yin H, Liang J, Li X. Analysis of changes in characteristics of severe retinopathy of prematurity patients after screening guidelines were issued in China. Retina. 2015;35(8):1674–9. https://doi.org/10.1097/IAE.0000000000000512.
Shah PK, Narendran V, Kalpana N, Gilbert C. Severe retinopathy of prematurity in big babies in India: history repeating itself? Indian J Pediatr. 2009;76(8):801–4. https://doi.org/10.1007/s12098-009-0175-1.
Adeli M, Bloom WR. Implementing Telemedicine visits in an underserved ophthalmology clinic in the COVID-19 era. J Prim Care Community Health. 2021;12:2150132721996278. https://doi.org/10.1177/2150132721996278.
Weaver DT, Murdock TJ. Telemedicine detection of type 1 ROP in a distant neonatal intensive care unit. J AAPOS. 2012;16(3):229–33. https://doi.org/10.1016/j.jaapos.2012.01.007.
Wang SK, Callaway NF, Wallenstein MB, Henderson MT, Leng T, Moshfeghi DM. SUNDROP: six years of screening for retinopathy of prematurity with telemedicine. Can J Ophthalmol. 2015;50(2):101–6. https://doi.org/10.1016/j.jcjo.2014.11.005.
Scruggs BA, Chan RVP, Kalpathy-Cramer J, Chiang MF, Campbell JP. Artificial intelligence in retinopathy of prematurity diagnosis. Transl Vis Sci Technol. 2020;9(2):5. https://doi.org/10.1167/tvst.9.2.5.
Daniel E, Quinn GE, Hildebrand PL, Ells A, Hubbard GB, Capone A, et al. Validated system for centralized grading of retinopathy of prematurity: telemedicine approaches to evaluating acute-phase retinopathy of prematurity (e-ROP) study. JAMA Ophthalmol. 2015;133(6):675–82. https://doi.org/10.1001/jamaophthalmol.2015.0460.
Quinn GE, Ells A, Capone A, Hubbard GB, Daniel E, Hildebrand PL, et al. Analysis of discrepancy between diagnostic clinical examination findings and corresponding evaluation of digital images in the telemedicine approaches to evaluating acute-phase retinopathy of prematurity study. JAMA Ophthalmol. 2016;134(11):1263–70. https://doi.org/10.1001/jamaophthalmol.2016.3502.
Chan RV, Patel SN, Ryan MC, Jonas KE, Ostmo S, Port AD, et al. The Global education network for retinopathy of prematurity (Gen-Rop): development, implementation, and evaluation of a novel tele-education system (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2015;113:T2.
Campbell JP, Swan R, Jonas K, Ostmo S, Ventura CV, Martinez-Castellanos MA, et al. Implementation and evaluation of a tele-education system for the diagnosis of ophthalmic disease by international trainees. AMIA Annu Symp Proc. 2015;2015:366–75.
Al-Khaled T, Mikhail M, Jonas KE, Wu WC, Anzures R, Amphonphruet A, et al. Training of Residents and Fellows in Retinopathy of Prematurity Around the World: An International Web-Based Survey. J Pediatr Ophthalmol Strabismus. 2019;56(5):282–7. https://doi.org/10.3928/01913913-20190717-01.
Campbell JP, Ryan MC, Lore E, Tian P, Ostmo S, Jonas K, et al. Diagnostic discrepancies in retinopathy of prematurity classification. Ophthalmology. 2016;123(8):1795–801. https://doi.org/10.1016/j.ophtha.2016.04.035.
Redd TK, Campbell JP, Brown JM, Kim SJ, Ostmo S, Chan RVP, et al. Evaluation of a deep learning image assessment system for detecting severe retinopathy of prematurity. Br J Ophthalmol. 2018:bjophthalmol-2018-313156. https://doi.org/10.1136/bjophthalmol-2018-313156.
Brown JM, Campbell JP, Beers A, Chang K, Ostmo S, Chan RVP, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136(7):803–10. https://doi.org/10.1001/jamaophthalmol.2018.1934.
Taylor S, Brown JM, Gupta K, Campbell JP, Ostmo S, Chan RVP, et al. Monitoring disease progression with a quantitative severity scale for retinopathy of prematurity using deep learning. JAMA Ophthalmol. 137(9):1022–8. https://doi.org/10.1001/jamaophthalmol.2019.2433.
Gupta K, Campbell JP, Taylor S, Brown JM, Ostmo S, Chan RVP, et al. A quantitative severity scale for retinopathy of prematurity using deep learning to monitor disease regression after treatment. JAMA Ophthalmol. 2019;137(9):1029–36. https://doi.org/10.1001/jamaophthalmol.2019.2442.
Campbell JP, Kim SJ, Brown JM, Ostmo S, RVP C, Kalpathy-Cramer J, et al. Evaluation of a deep learning-derived quantitative retinopathy of prematurity severity scale. Ophthalmology. 2021;128(7):1070–6. https://doi.org/10.1016/j.ophtha.2020.10.025This study showed that a deep learning derived vascular severity score correlates with zone, stage and extent of stage 3 ROP, and plus disease. This score may help physicians in the diagnosis of ROP.
Chen JS, Coyner AS, Ostmo S, Sonmez K, Bajimaya S, Pradhan E, et al. Deep learning for the diagnosis of stage in retinopathy of prematurity: accuracy and generalizability across populations and cameras. Ophthalmol Retina. 2021;5(10):1027–35. https://doi.org/10.1016/j.oret.2020.12.013.
Coyner AS, Chen JS, Singh P, Schelonka RL, Jordan BK, McEvoy CT, et al. Single-examination risk prediction of severe retinopathy of prematurity. Pediatrics. 2021;148(6). https://doi.org/10.1542/peds.2021-051772.
Campbell JP, Singh P, Redd TK, Brown JM, Shah PK, Subramanian P, et al. Applications of artificial intelligence for retinopathy of prematurity screening. Pediatrics. 2021;147(3). https://doi.org/10.1542/peds.2020-016618.
Palmer EA, Hardy RJ, Dobson V, Phelps DL, Quinn GE, Summers CG, et al. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005;123(3):311–8. https://doi.org/10.1001/archopht.123.3.311.
Hardy RJ, Good WV, Dobson V, Palmer EA, Phelps DL, Quintos M, et al. Multicenter trial of early treatment for retinopathy of prematurity: study design. Control Clin Trials. 2004;25(3):311–25. https://doi.org/10.1016/j.cct.2004.03.003.
Nagata M, Yamagishi N, Ikeda S. Summarized results of treatment of acute proliferative retinopathy of prematurity during the past 15 years in Tenri Hospital. Nippon Ganka Gakkai Zasshi. 1982;86(9):1236–44.
Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98(11):1628–40. https://doi.org/10.1016/s0161-6420(91)32074-8.
Good WV, Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–48 discussion 48-50.
Tasman W, Patz A, McNamara JA, Kaiser RS, Trese MT, Smith BT. Retinopathy of prematurity: the life of a lifetime disease. Am J Ophthalmol. 2006;141(1):167–74. https://doi.org/10.1016/j.ajo.2005.07.034.
Sankar MJ, Sankar J, Chandra P. Anti-vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database Syst Rev. 2018;1:CD009734. https://doi.org/10.1002/14651858.CD009734.pub3.
Mintz-Hittner HA. Retinopathy of Prematurity: intravitreal injections of bevacizumab: timing, technique, and outcomes. J AAPOS. 2016;20(6):478–80. https://doi.org/10.1016/j.jaapos.2016.10.002.
VanderVeen DK, Cataltepe SU. Anti-vascular endothelial growth factor intravitreal therapy for retinopathy of prematurity. Semin Perinatol. 2019;43(6):375–80. https://doi.org/10.1053/j.semperi.2019.05.011.
Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–15. https://doi.org/10.1056/NEJMoa1007374.
Hård AL, Hellström A. On safety, pharmacokinetics and dosage of bevacizumab in ROP treatment - a review. Acta Paediatr. 2011;100(12):1523–7. https://doi.org/10.1111/j.1651-2227.2011.02445.x.
Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394(10208):1551–9. https://doi.org/10.1016/S0140-6736(19)31344-3This randomized controlled trial showed the efficacy and safety of intravitreal ranibizumab in the treatment of ROP. Treatment success was at 80% with the use of ranibizumab 0.2mg.
Marlow N, Stahl A, Lepore D, Fielder A, Reynolds JD, Zhu Q, et al. 2-year outcomes of ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW extension study): prospective follow-up of an open label, randomised controlled trial. Lancet Child Adolesc Health. 2021;5(10):698–707. https://doi.org/10.1016/S2352-4642(21)00195-4.
Kaushal M, Razak A, Patel W, Pullattayil AK, Kaushal A. Neurodevelopmental outcomes following bevacizumab treatment for retinopathy of prematurity: a systematic review and meta-analysis. J Perinatol. 2021;41(6):1225–35. https://doi.org/10.1038/s41372-020-00884-9.
Tsai CY, Yeh PT, Tsao PN, Chung YE, Chang YS, Lai TT. Neurodevelopmental outcomes after bevacizumab treatment for retinopathy of prematurity: a meta-analysis. Ophthalmology. 2021 Jun;128(6):877–88. https://doi.org/10.1016/j.ophtha.2020.11.012This meta-analysis of eight studies showed that the risk of severe neurodevelopmental impairment was not increased in ROP patients after intravitreal bevacizumab treatment.
Wallace DK, Kraker RT, Freedman SF, Crouch ER, Bhatt AR, Hartnett ME, et al. Short-term outcomes after very low-dose intravitreous bevacizumab for retinopathy of prematurity. JAMA Ophthalmol. 2020;138(6):698–701. https://doi.org/10.1001/jamaophthalmol.2020.0334.
Stahl A, Krohne TU, Eter N, Oberacher-Velten I, Guthoff R, Meltendorf S, et al. Comparing alternative ranibizumab dosages for safety and efficacy in retinopathy of prematurity: a randomized clinical trial. JAMA Pediatr. 2018;172(3):278–86. https://doi.org/10.1001/jamapediatrics.2017.4838.
Xu Y, Zhang Q, Kang X, Zhu Y, Li J, Chen Y, et al. Early vitreoretinal surgery on vascularly active stage 4 retinopathy of prematurity through the preoperative intravitreal bevacizumab injection. Acta Ophthalmol. 2013;91(4):e304–10. https://doi.org/10.1111/aos.12055.
Kychenthal A, Dorta P. Vitrectomy after intravitreal bevacizumab (Avastin) for retinal detachment in retinopathy of prematurity. Retina. 2010;30(4 Suppl):S32–6. https://doi.org/10.1097/IAE.0b013e3181ca146b.
Nudleman E, Muftuoglu IK, Gaber R, Robinson J, Drenser K, Capone A, et al. Glaucoma after lens-sparing vitrectomy for advanced retinopathy of prematurity. Ophthalmology. 2018;125(5):671–5. https://doi.org/10.1016/j.ophtha.2017.11.009.
Nudleman E, Robinson J, Rao P, Drenser KA, Capone A, Trese MT. Long-term outcomes on lens clarity after lens-sparing vitrectomy for retinopathy of prematurity. Ophthalmology. 2015;122(4):755–9. https://doi.org/10.1016/j.ophtha.2014.11.004.
Choi J, Kim JH, Kim SJ, Yu YS. Long-term results of lens-sparing vitrectomy for progressive posterior-type stage 4A retinopathy of prematurity. Korean J Ophthalmol. 2012;26(4):277–84. https://doi.org/10.3341/kjo.2012.26.4.277.
Fierson WM. American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189–95. https://doi.org/10.1542/peds.2012-2996.
Lepore D, Quinn GE, Molle F, Orazi L, Baldascino A, Ji MH, et al. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2018;125(2):218–26. https://doi.org/10.1016/j.ophtha.2017.08.005.
Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, et al. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology. 2014;121(11):2212–9. https://doi.org/10.1016/j.ophtha.2014.05.015.
Banach MJ, Ferrone PJ, Trese MT. A comparison of dense versus less dense diode laser photocoagulation patterns for threshold retinopathy of prematurity. Ophthalmology. 2000;107(2):324–7; discussion 328. https://doi.org/10.1016/s0161-6420(99)00042-1.
Fielder A, Blencowe H, O'Connor A, Gilbert C. Impact of retinopathy of prematurity on ocular structures and visual functions. Arch Dis Child Fetal Neonatal Ed. 2015;100(2):F179–84. https://doi.org/10.1136/archdischild-2014-306207.
Kaiser RS, Trese MT, Williams GA, Cox MS. Adult retinopathy of prematurity: outcomes of rhegmatogenous retinal detachments and retinal tears. Ophthalmology. 2001;108(9):1647–53. https://doi.org/10.1016/s0161-6420(01)00660-1.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119(8):1110–8. https://doi.org/10.1001/archopht.119.8.1110.
Quinn GE, Dobson V, Hardy RJ, Tung B, Palmer EA, Good WV, et al. Visual field extent at 6 years of age in children who had high-risk prethreshold retinopathy of prematurity. Arch Ophthalmol. 2011;129(2):127–32. https://doi.org/10.1001/archophthalmol.2010.360.
Smith J, Shivitz I. Angle-closure glaucoma in adults with cicatricial retinopathy of prematurity. Arch Ophthalmol. 1984;102(3):371–2. https://doi.org/10.1001/archopht.1984.01040030289020.
VanderVeen DK, Bremer DL, Fellows RR, Hardy RJ, Neely DE, Palmer EA, et al. Prevalence and course of strabismus through age 6 years in participants of the Early Treatment for Retinopathy of Prematurity randomized trial. J AAPOS. 2011;15(6):536–40. https://doi.org/10.1016/j.jaapos.2011.07.017.
Ober RR, Palmer EA, Drack AV, Wright KW. Retinopathy of Prematurity. In Wright KW, Spiegel PH, Thompson LS. Handbook of pediatric retinal disease. Springer; 2006. p. 329.
Huang CY, Lien R, Wang NK, Chao AN, Chen KJ, Chen TL, et al. Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2018;256(3):479–87. https://doi.org/10.1007/s00417-017-3878-4.
Wu WC, Shih CP, Lien R, Wang NK, Chen YP, Chao AN, et al. Serum vascular endothelial growth factor after bevacizumab or ranibizumab treatment for retinopathy of prematurity. Retina. 2017;37(4):694–701. https://doi.org/10.1097/IAE.0000000000001209.
Park CH, Rahimy E, Shahlaee A, Federman JL. Telemedicine in ophthalmology. Retina Today. 2017:55–8. https://retinatoday.com/articles/2017-apr/telemedicine-in-phthalmology. Accessed July 2022
Valikodath N, Cole E, Chiang MF, Campbell JP, Chan RVP. Imaging in retinopathy of prematurity. Asia Pac J Ophthalmol (Phila). 2019;8(2):178–86. https://doi.org/10.22608/APO.201963.
Patel SN, Shi A, Wibbelsman TD, Klufas MA. Ultra-widefield retinal imaging: an update on recent advances. Ther Adv Ophthalmol. 2020;12:2515841419899495. https://doi.org/10.1177/2515841419899495.
Vinekar A, Rao SV, Murthy S, Jayadev C, Dogra MR, Verma A, et al. A novel, low-cost, wide-field, infant retinal camera, "neo": technical and safety report for the use on premature infants. Transl Vis Sci Technol. 2019;8(2):2. https://doi.org/10.1167/tvst.8.2.2.
Lee AC, Maldonado RS, Sarin N, O'Connell RV, Wallace DK, Freedman SF, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31(8):1470–82. https://doi.org/10.1097/IAE.0b013e31821dfa6d.
Campbell JP, Nudleman E, Yang J, Tan O, Chan RVP, Chiang MF, et al. Handheld optical coherence tomography angiography and ultra-wide-field optical coherence tomography in retinopathy of prematurity. JAMA Ophthalmol. 2017;135(9):977–81. https://doi.org/10.1001/jamaophthalmol.2017.2481.
Wintergerst MWM, Petrak M, Li JQ, Larsen PP, Berger M, Holz FG, et al. Non-contact smartphone-based fundus imaging compared to conventional fundus imaging: a low-cost alternative for retinopathy of prematurity screening and documentation. Sci Rep. 2019;9(1):19711. https://doi.org/10.1038/s41598-019-56155-x.
Patel TP, Aaberg MT, Paulus YM, Lieu P, Dedania VS, Qian CX, et al. Smartphone-based fundus photography for screening of plus-disease retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2019;257(11):2579–85. https://doi.org/10.1007/s00417-019-04470-4.
Sharma A, Goyal A, Bilong Y, Shah P, Banker A, Kumar N, et al. Comparison of a smartphone-based photography method with indirect ophthalmoscopic assessment in referable retinopathy of prematurity: a smart retinopathy of prematurity model pilot study. Ophthalmol Retina. 2019;3(10):911–2. https://doi.org/10.1016/j.oret.2019.06.006.
Acknowledgements
We would like to thank Andrew Nguyen and Yifan Jian for their help in obtaining the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
MFC was a Consultant for Novartis (Basel, Switzerland); received grant support from the National Institutes of Health (Bethesda, MD), the National Science Foundation (Arlington, VA), and Genentech (South San Francisco, CA); and was an equity owner in InTeleretina, LLC (Honolulu, HI). RVPC is a consultant for Alcon (Ft. Worth, TX).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Neonatology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsai, A.S.H., Acaba-Berrocal, L., Sobhy, M. et al. Current Management of Retinopathy of Prematurity. Curr Treat Options Peds 8, 246–261 (2022). https://doi.org/10.1007/s40746-022-00249-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-022-00249-8