Abstract
Introduction
This study assesses the accuracy of neutrophil activation markers, including neutrophil extracellular traps (NETs) and calprotectin, as biomarkers of disease activity in patients with established rheumatoid arthritis (RA). We also analyse the relationship between NETs and various types of therapies as well as their association with autoimmunity.
Methods
Observational cross-sectional study of patients with RA receiving treatment with biological disease-modifying antirheumatic drugs or Janus kinase inhibitors (JAK-inhibitors) for at least 3 months. Plasma calprotectin levels were measured using an enzyme-linked immunosorbent assay test kit and NETs by measuring their remnants in plasma (neutrophil elastase-DNA and histone-DNA complexes). We also assessed clinical disease activity, joint ultrasound findings and autoantibody status [reumatoid factor (RF), anti-citrullinated peptide/protein antibodies (ACPAs) and anti-carbamylated protein (anti-CarP)]. Associations between neutrophilic biomarkers and clinical or ultrasound scores were sought using correlation analysis. The discriminatory capacity of both neutrophilic biomarkers to detect ultrasound synovitis was analysed through receiver-operating characteristic (ROC) curves.
Results
One hundred fourteen patients were included. Two control groups were included to compare NET levels. The active control group consisted of 15 patients. The second control group consisted of 30 healthy subjects. Plasma NET levels did not correlate with clinical disease status, regardless of the clinic index analysed or the biological therapy administered. No significant correlation was observed between NET remnants and ultrasound synovitis. There was no correlation between plasma NET and autoantibodies. In contrast, plasma calprotectin positively correlated with clinical parameters (swollen joint count [SJC] rho = 0.49; P < 0.001, Clinical Disease Activity Index [CDAI] rho = 0.30; P < 0.001) and ultrasound parameters (rho > 0.50; P < 0.001). Notably, this correlation was stronger than that observed with acute phase reactants.
Conclusion
While NET formation induced by neutrophils may play a role in RA pathogenesis, our study raises questions about the utility of NET remnants in peripheral circulation as a biomarker for inflammatory activity. In contrast, this study strongly supports the usefulness of calprotectin as a biomarker of inflammatory activity in patients with RA.
Similar content being viewed by others
Why carry out this study? |
Classical acute phase reactants do not always reflect the synovitis in the patient and may be influenced by other factors. |
The search for new biomarkers of disease activity has been a growing field of research in recent years. |
Extensive research has reported that the innate immune system, especially the involvement of neutrophils, plays a central role in the initiation and perpetuation of rheumatoid arthritis (RA). |
The objective of this study is to determine the performance of neutrophil activation markers, including neutrophil extracellular traps (NETs) and calprotectin, as biomarkers of disease activity using clinical and joint ultrasound (US) parameters in patients with established RA. |
We also analyse the relationship between NETs and various types of therapies as well as their association with autoimmunity. |
What was learned from this study? |
While NET formation induced by neutrophils may play a role in RA pathogenesis, our study raises questions about the utility of NET remnants in peripheral circulation as a biomarker for inflammatory activity. |
This study strongly supports the usefulness of calprotectin as a biomarker of inflammatory activity in patients with RA. |
This study represents an important contribution to the growing body of research exploring the association among NETs, disease activity and autoantibodies in RA. |
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease characterized by synovial inflammation and cartilage and joint destruction [1]. Over the past few decades, the prognosis of this disease has improved significantly thanks to the adoption of a treat-to-target approach, early diagnosis and treatment, and the utilization of more effective antirheumatic drugs [2, 3]. Currently, close monitoring of the inflammatory disease activity in RA is strongly recommended using a therapeutic goal of achieving remission or low disease activity [2, 4]. In addition to clinical parameters, traditional acute phase reactants (APRs) such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) serum concentration are commonly employed as laboratory markers to assess disease activity in RA. These parameters are also integrated into composite disease activity indices like the Simplified Disease Activity Index (SDAI) or the Disease Activity Score 28 (DAS28) for longitudinal evaluation [5]. However, while commonly used, APRs may not always reflect the degree of active synovitis in patients with RA accurately.
Extensive research has demonstrated the significant involvement of adaptive immunity in the pathogenesis of RA. Systemic autoimmunity is found in most patients with RA, with the presence of characteristic autoantibodies such as rheumatoid factor (RF) or anti-citrullinated peptide/protein antibodies (ACPAs). However, mounting evidence indicates that the innate immune system, especially the involvement of neutrophils, plays a central role in the initiation and perpetuation of RA, both directly through its effects on the synovium and indirectly via its own inflammatory response and by modulating that of the adaptive immune system [6,7,8]. In recent years, increasing attention has been paid to the role of neutrophils and their products in RA. Calprotectin (S100A9/S100A8), a protein member of the S100 family which is secreted by activate neutrophils, seems to play a significant role in the inflammatory cascade in RA. Calprotectin is a significant proinflammatory factor of innate immunity and acts as an endogenous damage-associated molecular pattern (DAMP) through the activation of TLR4 [9]. Moreover, when secreted into the synovium, calprotectin subsequently enters peripheral blood circulation. Levels of calprotectin in serum or plasma have been found to correlate closely with inflammatory disease activity in patients with RA and therefore provide a more accurate reflection of the patient's clinical status than APRs [10,11,12,13].
In 2004, a mechanism of action was reported for neutrophils through the formation of neutrophil extracellular traps (NETs) [14]. NETs are extracellular structures that contain neutrophil DNA, histones and proteins and which are ejected into the extracellular medium in the form of a network. NETs provide an alternative defence mechanism and may play a crucial role in regulating the immune response and maintaining bodily homeostasis. They are formed through a process called NETosis, which can be triggered by various stimuli, including both infectious and sterile factors such as autoantibodies or immunocomplexes [15].
The role of NETs in RA has garnered significant interest in recent years. These inflammatory structures are believed to have both local and systemic effects, potentially contributing to the initiation and perpetuation of the disease through autoantigen exposure [16,17,18,19,20,21]. Furthermore, NETs have been identified as a source of specific autoantigens in RA, including ACPAs and anti-carbamylated protein (anti-CarP) antibodies [22,23,24,25,26]. Patients with RA demonstrate elevated NET formation compared to the general population, revealing NETs to be a promising potential biomarker for the disease [27]. However, the precise role of NETs in disease activity remains uncertain as studies evaluating their utility as a biomarker have produced conflicting results [17, 25, 26, 28].
The objective of this study is to determine the performance of neutrophil activation markers, including NETs and calprotectin, as biomarkers of disease activity using clinical and joint ultrasound (US) parameters in patients with established RA. The study also aims to examine the relationship between NETs and various types of therapies as well as their association with serum autoantibodies.
Methods
Design and Study Population
We performed an observational cross-sectional study. Patients with RA (ACR/EULAR 2010 criteria) [29] from our Arthritis Unit who had been undergoing treatment with biological disease-modifying antirheumatic drugs (DMARDs), tumour necrosis factor inhibitors (anti-TNF), monoclonal antibodies against IL-6 receptors (anti-IL6r) or Janus kinase inhibitors (JAKi) for at least 3 months were consecutively included, irrespective of their disease activity status, previous use of DMARDs (including biological therapies or JAKi) or concomitant treatment (such as with methotrexate or others). Patients who displayed signs of active infection or other clinical conditions that could impact the determination of neutrophil activation markers (calprotectin or NETs) or APR measurements, as determined by the researcher concerned, were excluded. Demographic data, disease duration, autoantibody status (RF, ACPAs and anti-CarP), radiological data (presence/absence of erosive disease) and information on previous biological therapy and concurrent treatments were collected.
Two control groups were included to assess NET levels. The first consisted of patients with RA [29] from our Arthritis Unit with high disease activity, defined as those with at least four inflamed joints, regardless of treatment or disease duration. The second control group was comprised of healthy subjects.
Measurement of Clinical Disease Activity
All patients received a clinical evaluation that included the counting of 28 swollen and tender joints (28SJC and 28TJC) as well as physician and patient self-reported global assessments (PhGA and PGA respectively) using visual analogue scales ranging from 0 to 10 along a centimetre scale. Subsequently, composite disease activity indices such as DAS28, SDAI and CDAI (Clinical Disease Activity Index) were calculated. Additionally, patients were asked to complete two questionnaires: the Health Assessment Questionnaire (HAQ) and Routine Assessment of Patient Index Data 3 (RAPID3).
Assessment of Biomarkers
Acute Phase Reactants
Blood samples were collected concurrently with the clinical assessment. ESR was measured using the Westergren method (normal value [NV] < 20 mm/h) and high-sensitivity C-reactive protein (hsCRP) plasma concentration using an immunoturbidimetric method measured with Siemens Atellica® Solution (lowest limit of detection: 0.02 mg/dl; NV < 0.4 mg/dl).
Plasma Calprotectin
Calprotectin was measured using an enzyme-linked immunosorbent assay (ELISA) test kit (CALPROLAB ALP [CALPRO], Menarini Diagnósticos S.A.) following the manufacturer's instructions. Briefly, 100 µl aliquots of each standard, control and diluted 1:20 sample in duplicate wells were incubated at room temperature for 40 min; three washings were performed, 100 µl of the conjugated enzyme was added and the plates were incubated at room temperature for another 40 min. After three further washes and the addition of the enzyme substrate, optical density values at 405 nm were determined using an ELISA reader. To reduce variation in calprotectin determinations, the whole procedure was performed in a Triturus Autoanalyzer; the coefficients of variation were 5% within and 13% between assays. This technique had already been used by our group for previous studies[11].
Assessment of NETs
NETs were indirectly determined by measuring their remnants in plasma. Neutrophil elastase-DNA (NE-DNA) and histone-DNA (H3-DNA) complex levels were determined using a tailor-made ELISA[30, 31]. A 96-well plate was coated with 100 µl of capture antibodies, anti-elastase (Millipore cat. no. 481001) and anti-histone H3 citrulline (Abcam cat. no. ab5103) at 2.5 µg/ml in sterile PBS. The ELISA plates were incubated overnight at 4 °C without agitation to allow the binding of capture antibodies. The next day, the wells were washed three times with PBS-Tween 20 (200 μl/well) and blocked with PBS-1% BSA (200 μl/well) at room temperature for 1 h. Again, three repeated washes with PBS-Tween 20 (200 μl/well) were performed. Diluted plasma (1:100, with PBS-1% BSA) was added to the wells (100 μl/well) and again incubated overnight at 4 °C but with agitation (100–150 rpm). Three washes with PBS-Tween 20 (200 μl/well) were performed to remove unbound NETs and other components. Mouse anti-dsDNA (Millipore cat no. MAB030) diluted 1:100 with PBS-1% BSA was added to the wells (100 μl/well) and incubated for 1 h, at room temperature, with agitation (100–150 rpm). The plates were washed three more times with PBS-Tween 20 (200 μl/well). Goat anti-mouse, HRP conjugate (Millipore cat no. AP127P) diluted 1:10,000 was added (100 μl/well) and incubated for 1 h at room temperature. The plates were washed five times with PBS-Tween 20 (200 μl/well). Then, TMB peroxidase substrate was added to each well (50 μl/well) and the plate was incubated in the dark for between 10 and 15 min. The reaction was stopped by the addition of 1 M HCl to each well (50 μl/well). Finally, absorbance was measured at 450 nm using a microplate photometer, and an OD index (OD each well/OD blank) was calculated for each well. The assay was performed in duplicate and mean values were calculated.
Assessment of Autoantibodies (RF, ACPAs and Anti-CarP)
Autoantibody status was measured in sera collected at enrolment in the study.
IgM RF was determined using a chemiluminescence assay (CIA) (QUANTA Flash®, Inova Diagnostics, CA). The assay's analytical measurement range (AMR) is from 0.3 to 490.0 IU/ml and the cut-off recommended by the manufacturer is < 5 IU/ml.
ACPA levels were determined by a tailor-made ELISA using a chimeric fibrin/filaggrin citrullinated peptide (anti-CFFCP1) as an antigen [32] biotinylated at its terminal amino group with a biotin derivative containing two polyethylene glycol (PEG) chains bound to neutravidin derivatized microtitre plates. This derivatization strategy allows for better antigenic exposure of the peptide in the ELISA plates.
Anti-CarP antibodies were also determined using a tailor-made ELISA test using carbamylated foetal calf serum (FCS-CarP) as the antigen and the non-homocitrullinated version of the protein as the control for the homocitrulline anti-FCS-CarP detected [33].
A positive cut-off value for the tests was defined as ≥ 11.5 AU/ml and ≥ 173.5 AU/ml for ACPAs (anti-CFFCP1) and anti-CarP antibodies (anti-FCS-CarP), respectively. A result was only considered positive and specific for citrulline and/or homocitrulline when the UA/ml values were higher than the respective cut-offs and the difference in OD values between native and post-translationally modified antigens was > 0.1. Further details of the ELISA techniques are provided in the Supplementary Material.
Imaging Biomarkers: Ultrasound Score
Sonographic assessments were carried out using high-sensitivity US equipment (MyLab9®; Esaote, Genoa, Italy), a longitudinal probe with a 10–14 MHz frequency range and a pulse repetition frequency of between 800 and 900 Hz. The joint musculoskeletal US findings were defined based on the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) definitions [34].
In accordance with the EULAR guidelines [35], synovial hypertrophy (SH) and intra-articular power Doppler (PD) signalling of 11 joints and tendons on each hand, including the proximal interphalangeal joints, metacarpophalangeal joints and wrists, were evaluated by a single experienced sonographer (A.P.). The sonographer was blinded to the results of the clinical joint examination. SH and PD signals were graded using a four-grade semi-quantitative scoring system (0 = no, 1 = mild, 2 = moderate and 3 = severe) according to the methodology of Szkudlarek et al. [36]. The highest grade of SH and PD signalling detected during the scans was selected as representative of each joint, respectively.
The PD score (sum of PD scores in all joints, range: 0–66), SH score (sum of SH scores in all joints, range: 0–66) and global score (sum of the PD and SH scores, range: 0–132) were calculated by adding up the scores for elementary lesions in each joint. To ensure strict criteria for defining US synovitis, only patients with SH grade ≥ 2 plus PD signal ≥ 1 were considered to have active synovitis [37]. This US assessment method had been previously employed in prior studies conducted by our group [11]. Clinically symptomatic joints were also evaluated to assess whether US synovitis was present. However, these joints were not used to calculate the sonographic score (PD score, SH score and global US score).
Statistical Analysis
Continuous data were presented as mean and standard deviation (SD) while categorical variables were given as absolute frequency with percentages. Groups were compared using parametric or nonparametric tests according to the distribution of the variables.
Correlation analysis (Spearman’s correlation coefficient) was used to assess the association between the different activity indices (clinical and US activity) and the neutrophil activation markers (NET and calprotectin plasma levels). To specifically investigate the relationship between calprotectin and the US parameters (including the US total score, SH score, and PD score), we utilized a linear regression model. This regression analysis was adjusted to account for potential confounding factors, with the selection of these variables guided solely by clinical criteria. Furthermore, similar models were constructed to assess the association among ESR, hsCRP and the US parameters. We also analysed the correlation between autoantibody levels and NETs. The performance of NETs (NE-DNA complexes and H3-DNA complexes) and calprotectin in the diagnosis of US synovitis was analysed using receiver-operating characteristic curves (ROC) with US synovitis yes/no (yes: SH grade ⩾ 2 plus PD signal ⩾ 1) as the gold standard. The ROC curves made it possible to identify the best cut-off point in terms of sensitivity, while specificity enabled us to calculate the area under the curve (AUC) as a measure of the overall discriminative power. The performance of ESR and hsCRP was also studied, and the three ROC curves were compared to determine which of the three parameters had the highest discriminatory power for the diagnosis of US synovitis. The test for the equality of the AUC uses an algorithm suggested by DeLong, DeLong and Clarke-Pearson (1988) [38]. Additionally, a logistic regression model was conducted to investigate the correlation between calprotectin and the presence of US synovitis (categorized as "yes" when SH grade ⩾ 2 plus PD signal ⩾ 1 were present). Similar to the linear regression model, these logistic regression models were adjusted for the same confounding variables.
Analysis was carried out using SPSS software (version 27.0, IBM Inc., Chicago, IL, USA) and Stata version 13.1 (StataCorp LP 4905 Lakeway Drive College Station, TX, USA).
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of the Hospital Clinic of Barcelona (reg. HCB20210783). Written informed consent was obtained from all patients before enrolment in the study.
Results
Demographic, Clinical and Therapeutic Characteristics
The study included 114 patients: 90.4% women; mean age 56 ± 11 years; mean disease duration 16 ± 20 years. Most patients (86%) were seropositive for either RF or ACPAs, 67.5% had erosive disease and 31.6% had extra-articular manifestations of RA. Fifty-six patients (49%) were receiving treatment with anti-IL6r (49 tocilizumab and 7 sarilumab), 28 (25%) were receiving JAKi (19 baricitinib and 9 tofacitinib) and 30 (26%) were receiving anti-TNF treatment (21 etanercept and 9 adalimumab). The mean duration of these treatments was 56 ± 58 months. Nearly half of the patients were receiving concomitant conventional DMARD treatment (methotrexate or leflunomide) (49.1%), and 44.7% were also receiving glucocorticoid treatment (mean prednisone equivalent dose 3.3 ± 2.8 mg/day) (Table 1).
As for disease activity, the mean CDAI was 12 ± 10; 64.3% of the patients were in remission and/or exhibited low disease activity according to CDAI criteria (Table 2). Regarding laboratory parameters, the mean hsCRP was 0.4 ± 1.3 mg/dl, the ESR was 14 ± 18 mm/h and the mean plasma calprotectin concentration was 0.99 ± 1.5 μg/ml. When comparing the parameters between the groups, we found that the classical APRs (both hsCRP and ESR) were significantly lower in the group of patients treated with anti-IL6r. However, we did not find statistically significant differences in plasma calprotectin levels between the groups (Table 2).
Ninety-nine patients were studied with US, of which 66 (67%) were active according to US criteria (SH score ≥ 2 and PD score ≥ 1). The mean scores for SH, PD and total US activity were 6.6 ± 6.4, 4.1 ± 5.5 and 11 ± 12, respectively.
When differentiating by treatment group, we found no differences between the patients in terms of the different clinical and US parameters of disease activity, except for DAS28, which was lower in the group of patients treated with anti-IL6r (Table 2).
Two control groups were included to compare NET levels. The active control group consisted of 15 patients: 93% female; mean age 54 ± 12 years. The mean disease duration was 13 ± 15 years. All these patients were seropositive, 67% had erosive disease, and 13% had extra-articular manifestations of RA. Eleven patients (73%) were not receiving targeted therapy, neither biological DMARDs nor JAKi. Four (27%) were receiving treatment with anti-IL6r, and three (20%) with rituximab. At the time of inclusion, 73% were receiving concomitant glucocorticoid therapy (mean prednisone equivalent dose 7.0 ± 7.8 mg/day). The second control group consisted of 30 healthy subjects (mean age 47 ± 10 years, 73% women).
NET Plasma Levels in RA According to the Antirheumatic Drug Group
We analysed the levels of circulating NET remnants, measured as DNA-NE complexes and DNA-H3 complexes. Plasma levels of neither NET remnant showed statistically significant differences between the treatment groups (anti-Il6r, JAKi and anti-TNF) (Table 3).
Since the assessment of NETs is a novel and non-standardized technique, we included a group of healthy subjects and a group of patients with highly active RA, as previously defined, as negative and positive controls, respectively. Even though the active patient group exhibited higher levels of NETs than the main group and the healthy subject group, this difference did not reach statistical significance (Table 3).
Association Between NETs and Plasma Calprotectin with Clinical and Ultrasonographic Disease Activity
We analysed the correlation between neutrophil activation markers (calprotectin and NET levels) and clinical and US activity parameters as well as APRs.
We found that NET levels did not show a correlation with any of the clinical and US activity parameters analysed, either as a single group (Table 4) or when considering the different treatment groups separately (Table 5).
Plasma calprotectin showed a moderate correlation with clinical parameters (SJC rho = 0.49, PhGA rho = 0.39, CDAI rho = 0.30; P < 0.001) and US parameters (rho > 0.50; P < 0.001). This correlation was stronger than that observed with APR (Table 4). To further examine the relationship between plasma calprotectin levels and US parameters, we conducted a linear regression model. This analysis was adjusted to account for potential confounding factors such as gender, smoking status, disease duration, use of glucocorticosteroids and drug therapy.
Our findings revealed a positive association between plasma calprotectin and SH and PD scores. Specifically, each increase in plasma calprotectin units corresponded to an increase of 2.14 units in HS score (95% confidence interval: 0.71–3.58; P value = 0.004) and 1.66 units in PD score (95% confidence interval: 0.35–2.7; P value = 0.013). However, no significant association was observed between plasma calprotectin levels and the total US score. Similar models were applied to ESR and hsCRP but failed to demonstrate a significant association (refer to Supplementary Material for details).
When we analysed the correlations by treatment groups, we found that calprotectin showed a moderate or strong correlation with US parameters, unlike classical APR; this is especially relevant for the group of patients treated with IL6 inhibitors (Table 5).
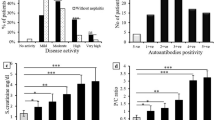
When analysing the discriminatory capacity by the AUC of both the neutrophil biomarkers of the presence of active US synovitis, calprotectin demonstrated an excellent capacity (Fig. 1) but plasma NETs did not (Figs. 2 and 3). This discriminatory capacity of plasma calprotectin was reflected by an AUC of 0.812 (95% confidence interval [CI]: 0.729–0.894), in contrast to the lower discriminatory capacity of hsCRP and ESR, with AUCs of 0.598 (0.531–0.665) and 0.560 (0.444–0.676), respectively (Fig. 1a). When we analysed the treatment groups separately, we found that these differences were maintained (Fig. 1b, c and e).
ROC curves of plasma calprotectin, hsCRP and ESR vs ultrasound synovitis according to type of treatment. a Main group (n = 114); b anti-Il6r (n = 56); c JAKi (n = 28); d anti-TNF (n = 30). Anti-Il6r monoclonal antibodies against IL-6 receptors; JAKi JAK inhibitors; anti-TNF tumour necrosis factor inhibitors; hsCRP high sensitivity C-reactive protein; ESR erythrocyte sedimentation rate; ROC receiver-operating characteristic
ROC curves of NE-DNA complexes, hsCRP and ESR vs ultrasound synovitis according to type of treatment. a Main group (n = 114); b anti-Il6r (n = 56); c JAKi (n = 28); d anti-TNF (n = 30). Anti-Il6r monoclonal antibodies against interleukin-6 receptors; JAKi JAK inhibitors; anti-TNF tumour necrosis factor inhibitors; NE-DNA complex neutrophil elastase-DNA complexes (NETs); hsCRP high sensitivity C-reactive protein; ESR erythrocyte sedimentation rate; ROC receiver-operating characteristic
ROC curves of H3-DNA complexes, hsCRP and ESR vs ultrasound synovitis according to type of treatment. a Main group (n = 114); b anti-Il6r (n = 56); c JAKi (n = 28); d anti-TNF (n = 30). Anti-Il6r monoclonal antibodies against interleukin-6 receptors; JAKi Janus kinase inhibitors; anti-TNF tumour necrosis factor inhibitors; H3-DNA complex histone-DNA complexes (NETs); hsCRP high-sensitivity C-reactive protein; ESR erythrocyte sedimentation rate; ROC receiver-operating characteristic
Furthermore, a logistic regression model was employed to examine the association between calprotectin and the presence of US synovitis (categorized as "yes" when SH grade ⩾ 2 plus PD signal ⩾ 1 was present). Using a calprotectin level of 0.4 mcg/dl as the cut-off point, our analysis indicated that patients with plasma calprotectin values > 0.4 mcg/dl faced an 11-fold higher risk of being identified as active via ultrasound compared to those with lower values (OR 11.31; 95% CI 3.18–40.17; P < 0.001) (refer to Supplementary Material for details).
Correlation Results Between NETs and Autoantibody Levels
We analysed potential correlations between NET levels and autoantibodies (ACPAs, anti-CarP antibodies and RF). However, our results showed no correlations, even when autoantibody status was categorized into different quartiles (data not shown). We also did not find any differences in plasma NET levels when we classified patients as seropositive or seronegative for each of the autoantibodies analysed (Table 6).
Correlation Results Between Calprotectin and ACPA Status
We have analysed whether the correlation of calprotectin with clinical and ultrasound parameters of disease activity differs based on ACPA status (ACPA positive vs ACPA negative).
We have observed that patients who are ACPA positive maintain a strong correlation between plasma calprotectin levels and indices of clinical disease activity. However, in the ACPA-negative patient group, this correlation diminishes. On the other hand, when analysing the correlation with US parameters, we did find any differences between the two subgroups (Table 7).
Discussion
One of the main objectives of the present study was to assess the utility of plasma NET remnants as a parameter for evaluating clinical disease activity in patients with established RA. However, our findings demonstrated that plasma NET levels did not reflect the inflammatory disease status in our patients, regardless of the specific targeted therapy group analysed. Furthermore, we investigated the potential relationship between NET remnants and US-detected synovitis, but no significant association was observed. These results are consistent with previous studies that also failed to find a significant association between peripheral blood levels of NETs and disease activity [17, 39, 40]. However, some studies have shown an association with clinical disease activity, but those studies are heterogeneous, included limited numbers of patients and the associations described are weak and only have a few of the activity parameters analysed [21, 25, 26, 28, 41, 42].
The lack of correlation between NETs and inflammatory disease activity in our cohort may be attributed to the inclusion of patients undergoing biological or JAKi treatment with low disease activity. Nevertheless, even in our control group consisting of patients with high disease activity, NET levels were only slightly elevated and there were no significant differences compared to the main group. These findings indicate very strongly that NET remnants cannot be considered reliable biomarkers of disease activity in patients with RA, particularly those receiving targeted treatment. It is important to note, however, that the observed low levels of NETs in our patients may be influenced by the effect of biologics or JAKi, as previous research has reported reductions in NET formation following different treatments with biological drugs [28, 41].
The involvement of NETs in the pathogenesis of RA and their potential as a antigenic source and autoantibodies has been suggested [16, 22, 43]. Therefore, in this study, we aimed to determine the association between plasma NETs and the characteristic autoantibodies seen in RA. We simultaneously measured the levels of RF, ACPAs and anti-CarP antibodies in the same blood samples used for analysing NET levels. We did not find any correlation between the titres of autoantibodies and the levels of NETs in peripheral blood. Low levels of NETs together with the effect of biological therapy may affect the possible relationship between antibody levels and NET remnants in our cohort. One study found an association between ACPA titres and serum NET remnants (myeloperoxidase-DNA and NE-DNA complexes) in patients with RA, but only in those patients with extremely high ACPA levels [24]. The discrepancies observed between those findings and our study may be attributed to factors such as geographic origin, disease duration, methodological issues in NET determination or differences in drug therapies among the study populations.
The lack of association of plasma NET remnants and autoantibody levels in our study does not rule out a role for NETs in autoantibody production in RA as a consequence of higher antigenic exposition. Several in vitro studies corroborate the role of NETs in autoantibody production and the idea that autoantibodies stimulate neutrophils to form NETs [16, 22, 43]. In vitro studies have also demonstrated that neutrophils from patients with RA exhibit increased spontaneous NET formation, which correlates with levels of ACPAs [17, 22]. One major factor that predisposes patients to exacerbated NETosis is the proinflammatory environment present in RA-affected tissues. This inflammatory milieu, generated by cells such as synovial fibroblasts, dendritic cells and macrophages releasing proinflammatory cytokines like TNF-α, IL-1β and interferon-gamma (IFN-γ), can activate neutrophils and promote their migration to inflamed joints. Once there, the neutrophils are continuously stimulated by the proinflammatory environment, triggering the release of NETs. Exposure of neutrophils to sera from patients with RA, especially those with elevated ACPA and RF levels, as well as the inflammatory cytokines IL-17A and TNF, induces NET formation in isolated RA neutrophils, which are more susceptible to NET formation than those from healthy individuals [22]. NETs are also an important source of carbamylated proteins in patients with RA [44]. Anti-CarP antibodies are frequently present in RA and are associated with increased joint destruction, mortality and interstitial lung disease [33, 45]. These antibodies can activate osteoclasts and contribute to bone resorption, thus suggesting a causal relationship with bone damage in RA [44]. Moreover, the specific types of NETs involved in RA pathogenesis warrant further investigation. While some studies have identified specific NET components, such as citrullinated proteins, others have highlighted the presence of histones or antimicrobial peptides. Understanding the heterogeneity of NETs and their contribution to disease processes is crucial if we are to gain deeper insight into the complex mechanisms of RA.
Overall, the induction of NETs by autoantibodies, the association between blood NET levels and ACPA titres and the variations observed in different experimental settings together emphasize the intricate nature of the involvement of NETs in RA pathogenesis. Further research is needed to elucidate the specific subtypes and functional roles of NETs in the context of disease progression and treatment response.
Additionally, we examined a well-known neutrophil activity marker: calprotectin. Our results confirm that calprotectin is a good biomarker of disease activity in patients with RA, showing a correlation with activity measured by clinical and US parameters. It is a promising biomarker with potential use in monitoring patients, especially those using treatments that modify the levels of classical APR, such as anti-IL6r [46, 47] or JAKi [46, 47]. These results are in line with the existing literature. Previous studies have indicated that this protein reflects a patient's clinical status more accurately than APRs [10,11,12,13], including clinical remission or low disease activity. Furthermore, calprotectin serves as a prognostic biomarker for radiographic progression [48], a marker of therapeutic response to specific targeted DMARDs [20], and it has proved useful in predicting disease flares in seemingly controlled patients [49]. A recent publication by Sejersen et al. explored the relationship between calprotectin levels and autoantibody-characterized subgroups [50]. They described association between circulating calprotectin and inflammation in patients who are ACPA positive but not in patients with RA who are ACPA negative. In line with this research, our analysis of the cohort, stratified based on autoantibody status, revealed a notable correlation between calprotectin and clinical parameters in patients with RA who are ACPA positive but not in patients who are ACPA negative. However, interestingly, we did not observe any significant differences in ultrasound (US) parameters. It is important to note that the sample size in the seronegative subgroup in our cohort was small.
The present study has some limitations. First, the methodology used to assess NETs relied on an ELISA that detects proteins expressed in NETs but which are not exclusive to these structures. This indirect approach may have introduced variability and potential bias into the results. Another limitation of our study was its cross-sectional design, which restricted our ability to establish causality or observe changes in NET levels over time. Additionally, the patient cohort in our study consisted of individuals with long-standing RA who were undergoing targeted therapies. These treatments may influence the levels of NETs, potentially leading to lower levels than those observed in patients who were naïve to treatment. It is essential to acknowledge the potential confounding effect of medications on NET levels and to consider studying patients at different disease stages to better understand the dynamics of NETs in RA.
Despite these limitations, this study constitutes a significant contribution to the expanding body of research investigating the interplay among NETs, disease activity and autoantibodies in RA. However, based on the results of this study, we cannot advocate for the routine clinical measurement of NETs to monitor disease activity. Conversely, our findings solidify the notion that calprotectin serves as an exceptionally valuable biomarker for assessing inflammatory activity (both clinical and ultrasonographic). It plays a pivotal role, particularly in evaluating patients undergoing treatments that inhibit the production of classical RFA, such as rIL6 antagonists or JAKi.
Conclusion
NET formation induced by neutrophils may play a potential role in RA, but our results question the utility of NET remnants in peripheral circulation as a biomarker of inflammatory activity in this disease. No association between NETS and clinical and US disease activity or with autoantibodies was observed. By contrast, our study clearly confirms the usefulness of blood calprotectin, a protein derived from neutrophil activation, as a biomarker of inflammatory activity and local synovitis in patients with RA, thereby confirming the results of previous studies.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110: 102400.
van Vollenhoven R. Treat-to-target in rheumatoid arthritis—are we there yet? Nat Rev Rheumatol. 2019;15:180–6.
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:S685–99.
Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75:3–15.
Aletaha D, Smolen JS. The definition and measurement of disease modification in inflammatory rheumatic diseases. Rheumatic Disease Clinics of North America. Rheum Dis Clin North Am. 2006;32(1):9–44.
Cecchi I, Arias de la Rosa I, Menegatti E, Roccatello D, Collantes-Estevez E, Lopez-Pedrera C, et al. Neutrophils novel key players in rheumatoid arthritis. current and future therapeutic targets. Autoimmun Rev. 2018;17:1138–49.
Zhang L, Yuan Y, Xu Q, Jiang Z, Chu CQ. Contribution of neutrophils in the pathogenesis of rheumatoid arthritis. J Biomed Res. 2020;34:86.
O’Neil LJ, Kaplan MJ. Neutrophils in rheumatoid arthritis: breaking immune tolerance and fueling disease. Trends Mol Med. 2019;25:215–27.
Inciarte-Mundo J, Frade-Sosa B, Sanmartí R. From bench to bedside: calprotectin (S100A8/S100A9) as a biomarker in rheumatoid arthritis. Front Immunol. 2022;13:1001025.
Inciarte-Mundo J, Victoria Hernández M, Ruiz-Esquide V, Raquel Cabrera-Villalba S, Ramirez J, Cuervo A, et al. Serum calprotectin versus acute-phase reactants in the discrimination of inflammatory disease activity in rheumatoid arthritis patients receiving tumor necrosis factor inhibitors. Arthritis Care Res (Hoboken). 2016;68:899–906.
Frade-Sosa B, Ponce A, Inciarte-Mundo J, Morlà R, Ruiz-Esquide V, Macías L, et al. Plasma calprotectin as a biomarker of ultrasound synovitis in rheumatoid arthritis patients receiving IL-6 antagonists or JAK inhibitors. Ther Adv Musculoskelet Dis. 2022;14:1759720X2211141.
Inciarte-Mundo J, Hernández MV, Ruiz-Esquide V, Cabrera-Villalba SR, Ramirez J, Cuervo A, et al. Serum Calprotectin more accurately discriminates the inflammatory disease activity of rheumatoid arthritis patients receiving tnf inhibitors than acute phase reactants. Arthritis Care Res (Hoboken). 2015;68:899–906.
Inciarte-Mundo J, Ruiz-Esquide V, Hernández MV, Cañete JD, Cabrera-Villalba SR, Ramirez J, et al. Calprotectin more accurately discriminates the disease status of rheumatoid arthritis patients receiving tocilizumab than acute phase reactants. Rheumatology (Oxford). 2015;54:2239–43.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5.
Apel F, Zychlinsky A, Kenny EF. The role of neutrophil extracellular traps in rheumatic diseases. Nat Rev Rheumatol. 2018;14(8):467–75.
Song W, Ye J, Pan N, Tan C, Herrmann M. Neutrophil extracellular traps tied to rheumatoid arthritis: points to ponder. Front Immunol. 2020;11: 578129.
Wang W, Peng W, Ning X. Increased levels of neutrophil extracellular trap remnants in the serum of patients with rheumatoid arthritis. Int J Rheum Dis. 2018;21:415–21.
Barnado A, Crofford LJ, Oates JC. At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J Leukoc Biol. 2016;99:265–78.
Berthelot JM, Le Goff B, Neel A, Maugars Y, Hamidou M. NETosis: at the crossroads of rheumatoid arthritis, lupus, and vasculitis. Joint Bone Spine. 2017;84(3):255–62.
Zhao J, Jiang P, Guo S, Schrodi SJ, He D. Apoptosis, autophagy, NETosis, necroptosis, and pyroptosis mediated programmed cell death as targets for innovative therapy in rheumatoid arthritis. Front Immunol. 2021;12: 809806.
Pérez-Sánchez C, Ruiz-Limón P, Aguirre MA, Jiménez-Gómez Y, Arias-de la Rosa I, Ábalos-Aguilera MC, et al. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in rheumatoid arthritis patients. J Autoimmun. 2017;82:31–40.
Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40.
O’Neil LJ, Oliveira CB, Wang X, Navarrete M, Barrera-Vargas A, Merayo-Chalico J, et al. Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis. Ann Rheum Dis. 2023;82(5):630–8.
Wu S, Peng W, Liang X, Wang W. Anti-citrullinated protein antibodies are associated with neutrophil extracellular trap formation in rheumatoid arthritis. J Clin Lab Anal. 2021;35(3): e23662.
Rezende Oliveira S, Almeida A, de Arruda J, Henriques Schneider A, Florindo Carvalho V, Cavalcante Machado C, Ice Dias Corrê J, et al. Are neutrophil extracellular traps the link for the cross-talk between periodontitis and rheumatoid arthritis physiopathology? Rheumatology (Oxford). 2021;61:174–84.
Kaneko C, Kobayashi T, Ito S, Sugita N, Murasawa A, Nakazono K, et al. Circulating levels of carbamylated protein and neutrophil extracellular traps are associated with periodontitis severity in patients with rheumatoid arthritis: a pilot case-control study. PLoS ONE. 2018;13(2): e0192365.
Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16(3):R122.
Luque-Tévar M, Perez-Sanchez C, Patiño-Trives AM, Barbarroja N, Arias de la Rosa I, Abalos-Aguilera MC, et al. Integrative clinical, molecular, and computational analysis identify novel biomarkers and differential profiles of anti-TNF response in rheumatoid arthritis. Front Immunol. 2021;12:631662.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Byrd AS, Carmona-Rivera C, O’Neil LJ, Carlucci PM, Cisar C, Rosenberg AZ, et al. Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aav5908.
Seto N, Torres-Ruiz JJ, Carmona-Rivera C, Pinal-Fernandez I, Pak K, Purmalek MM, et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.134189.
Pérez ML, Gómara MJ, Ercilla G, Sanmartí R, Haro I. Antibodies to citrullinated human fibrinogen synthetic peptides in diagnosing rheumatoid arthritis. J Med Chem. 2007;50:3573–84.
Castellanos-Moreira R, Rodríguez-García SC, Gomara MJ, Ruiz-Esquide V, Cuervo A, Casafont-Solé I, et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease. Ann Rheum Dis. 2020;79:587–94.
Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Guidelines for musculoskeletal ultrasound in rheumatology [Internet]. Ann Rheum Dis Ann Rheum Dis. 2001;60(7):641–9.
Wakefield RJ, Balint PV, Szkudlarek M, Filippucci E, Backhaus M, D’Agostino MA, Sanchez EN, Iagnocco A, Schmidt WA, Bruyn GA, Kane D, O’Connor PJ, Manger B, Joshua F, Koski J, Grassi W, Lassere MN, Swen N, Kainberger F, Klauser A, Ostergaard M, Brown AK, CPO 7 SIGroup. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2006;33:440.
Szkudlarek M, Court-Payen M, Jacobsen S, Klarlund M, Thomsen HS, Østergaard M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48:955–62.
Ramírez J, Ruíz-Esquide V, Pomés I, Celis R, Cuervo A, Hernández MVMV, et al. Patients with rheumatoid arthritis in clinical remission and ultrasound-defined active synovitis exhibit higher disease activity and increased serum levels of angiogenic biomarkers. Arthritis Res Ther. 2014;16(1):R5.
DeLong E, DeLong D, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Wang X, Wang H, Lv X, Wang X. Original article DMARDs combined with acupuncture therapy to treat RA: study of effects on dsDNA/NETs level and mechanism analysis. Int J Clin Exp Med. 2020;13:5059–67.
Bach M, Moon J, Moore R, Pan T, Nelson JL, Lood C. A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol. 2020;72:47–56.
Ruiz-Limón P, Ortega R, Arias I, de la Rosa M, Abalos-Aguilera DC, Sanchez CP, Gomez YJ, et al. Tocilizumab improves the proatherothrombotic profile of rheumatoid arthritis patients modulating endothelial dysfunction, NETosis, and inflammation. Transl Res. 2017;183:87–103.
Fagerhol MK, Johnson E, Tangen J, Hollan I, Mirlashari MR, Nissen-Meyer LSH, et al. NETs analysed by novel calprotectin-based assays in blood donors and patients with multiple myeloma or rheumatoid arthritis: a pilot study. Scand J Immunol. 2020;91(5): e12870.
Nakabo S, Ohmura K, Akizuki S, Murakami K, Nakashima R, Hashimoto M, et al. Activated neutrophil carbamylates albumin via the release of myeloperoxidase and reactive oxygen species regardless of NETosis. Mod Rheumatol. 2020;30:345–9.
O’Neil LJ, Barrera-Vargas A, Sandoval-Heglund D, Merayo-Chalico J, Aguirre-Aguilar E, Aponte AM, et al. Neutrophil-mediated carbamylation promotes articular damage in rheumatoid arthritis. Sci Adv. 2020. https://doi.org/10.1126/sciadv.abd2688.
Trouw LA, Rispens T, Toes REM. Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis. Nat Rev Rheumatol. 2017;13:331–9.
Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum. 2011;63:43–52.
Frade-Sosa B, Ponce A, Ruiz-Esquide V, García-Yébenes MJ, Morlá R, Sapena N, et al. High sensitivity C reactive protein in patients with rheumatoid arthritis treated with antibodies against IL-6 or Jak inhibitors: a clinical and ultrasonographic study. Diagnostics (Basel). 2022;12(1):182.
Turina MC, Sieper J, Yeremenko N, Conrad K, Haibel H, Rudwaleit M, et al. Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis. 2014;73:1–3.
Inciarte-Mundo J, Ramirez J, Hernández MV, Ruiz-Esquide V, Cuervo A, Cabrera-Villalba SR, et al. Calprotectin strongly and independently predicts relapse in rheumatoid arthritis and polyarticular psoriatic arthritis patients treated with tumor necrosis factor inhibitors: a 1-year prospective cohort study. Arthritis Res Ther. 2018;20(1):275.
Sejersen K, Weitoft T, Knight A, Lysholm J, Larsson A, Rönnelid J. Serum calprotectin correlates stronger with inflammation and disease activity in ACPA positive than ACPA negative rheumatoid arthritis. Rheumatology (Oxford). 2023;4:kead641.
Acknowledgements
The authors acknowledge the assistance provided by the nurses Nuria Sapena and Marta Bassas from the Rheumatology department, the assistance with statistics provided by Fernando Alonso (Spanish Society of Rheumatology) and technical advice from Toffa Evans.
Funding
This study was partially supported by a donation from Openbank through its “Open Solidario” program. This work was partially supported by a grant to I.H. (PID2021-122216OB-I00) funded by the Spanish Ministry of Economy, Industry and Competitiveness at the European Regional Development Fund. The journal’s Rapid Service Fee will be funded by the authors.
Author information
Authors and Affiliations
Contributions
Beatriz Frade-Sosa: Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Writing – original draft; Writing – review & editing. Andrés Ponce: Methodology; Project administration; review & editing. Estíbaliz Ruiz-Ortiz: Formal analysis; Investigation; Methodology; Writing – review & editing. Noemí de Moner: Formal analysis; Investigation; Methodology; Writing – review & editing. María J. Gómara: Formal analysis; Investigation; Methodology; Writing – review & editing. Ana Belén Azuaga: Writing – review & editing. Juan Camilo Sarmiento-Monroy: Writing – review & editing. Rosa Morlà: Writing – review & editing. Virginia Ruiz-Esquide Writing – review & editing. Laura Macías: Writing – review & editing. Nuria Sapena: Writing – review & editing. Lola Tobalina: Writing – review & editing. Julio Ramirez: Writing – review & editing. Juan D. Cañete: Writing – review & editing. Jordi Yague: Investigation; Writing – review & editing. Josep M. Augé: Investigation; Writing – review & editing. José A. Gómez-Puerta: Writing – review & editing. Odette Viñas: Formal analysis; Investigation; Methodology; Writing – review & editing. Isabel Haro: Formal analysis; Investigation; Methodology; Writing – review & editing. Raimon Sanmartí: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
Beatriz Frade-Sosa received support for attending meetings and/or speaker honoraria from Pfizer, AbbVie, Lilly, BMS, Galápagos, Sandoz and GSK. Laura Macías received support for attending meetings and/or travel from Diasorin in 19 October (LabClin Congress). Juan Camilo Sarmiento-Monroy received support for attending meetings and/or speaker honoraria from Pfizer, AbbVie, Lilly, BMS, Galápagos, Astrazeneca and GSK. Jose A. Gómez-Puerta received speaker honoraria from AbbVie, BMS, Galápagos, GSK, Lilly, Pfizer, Sanofi and Roche. Raimon Sanmartí received speaker honoraria and/or investigation grants from AbbVie, BMS, Gebro-Pharma, Lilly, MSD, Pfizer, Sandoz. Sanofi and Roche. Andrés Ponce, Estíbaliz Ruiz, Noemi de Moner, María J Gómara, Ana Belén Azuaga, Rosa Morlà, Virginia Ruiz-Esquide, Nuria Sapena, Lola Tobalina, Julio Ramirez, Juan D Cañete, Jordi Yague, Josep M. Augé, Odette Viñas and Isabel Haro declare they have no conflicts of interest. No pharmaceutical companies have participated or influenced the development of this manuscript.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of the Hospital Clinic of Barcelona (Reg. HCB20210783). Informed consent was obtained from all patients before enrolment in the study.
Additional information
Prior Presentation Frade-Sosa B, Ponce Fernandez A, Ruiz-Ortiz E, et al. AB0317 Plasma nets levels in established rheumatoid arthritis patients receiving biological or jak inhibitor therapy and association with disease activity. Annals of the Rheumatic Diseases 2023; 82:1342-1343.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Frade-Sosa, B., Ponce, A., Ruiz-Ortiz, E. et al. Neutrophilic Activity Biomarkers (Plasma Neutrophil Extracellular Traps and Calprotectin) in Established Patients with Rheumatoid Arthritis Receiving Biological or JAK Inhibitors: A Clinical and Ultrasonographic Study. Rheumatol Ther (2024). https://doi.org/10.1007/s40744-024-00650-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40744-024-00650-9