Abstract
Introduction
Microvascular manifestations constitute a subtype of antiphospholipid syndrome, and those patients have relatively poor prognoses, so it is important to find markers for microvascular manifestations. This study was conducted to explore whether serum calprotectin could be a predictor of microvascular manifestations in antiphospholipid antibody (aPL)-positive patients.
Methods
Consecutive patients with persistent aPL positivity referred to Peking Union Medical College Hospital and age- and sex-matched health controls (HCs) were included. Microvascular manifestations included antiphospholipid syndrome (APS) nephropathy, livedo reticularis, valvular lesions, non-stroke central nervous system manifestations, myocarditis, catastrophic APS, and other microvascular manifestations confirmed by pathology, imaging, or clinical diagnosis. Calprotectin was measured by an enzyme-linked immunosorbent assay (ELISA). The cutoff value was defined as mean + 2 standard deviations of HCs. Multivariable logistic regression analysis was used to analyze risk factors. Pearson correlation analysis was used to detect the correlation between calprotectin and other laboratory data.
Results
Of the 466 patients included in the study, 281 (60.3%) patients met the 2006 Sydney Revised Classification Criteria; among the latter, 77.2% were patients with primary APS. The mean age was 39.10 ± 13.05 years old, and 77.0% were female. Thirty-eight age- and sex-matched HCs were included in the study. Serum calprotectin levels were increased in aPL-positive patients compared with HCs (649.66 ± 240.79 vs 484.62 ± 149.37 ng/ml, p < 0.001), and were increased in patients with microvascular manifestations compared with patients without (693.03 ± 271.90 vs 639.43 ± 232.06 ng/ml, p = 0.044). The cutoff value was 783.36 ng/ml. Ninety-three patients (20.0%) were positive for calprotectin. Calprotectin positivity was independently associated with microvascular manifestations (odds ratio [OR] 1.90, 95% confidence interval [CI] 1.07–3.36) and platelet count (PLT) < 100 (OR 2.04, 95% CI 1.08–3.88). Age (OR 0.98, 95% CI 0.96–1.00), systemic lupus erythematosus (OR 2.08, 95% CI 1.15–3.75), calprotectin positivity (OR 1.83, 95% CI 1.02–3.26), hypertension (OR 2.73, 95% CI 1.36–5.45), hemolytic anemia (OR 2.66, 95% CI 1.13–6.23), and anti-β2GPI antibodies (OR 2.06, 95% CI 1.11–3.83) could independently predict microvascular manifestations in aPL-positive patients. Serum calprotectin negatively correlated with PLT (R = − 0.101, p = 0.031).
Conclusion
Serum calprotectin levels are increased in aPL-positive patients and could be a potential predictor of microvascular manifestations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Microvascular manifestations constitute a subtype of antiphospholipid syndrome and those patients have relatively poor prognoses. |
We hypothesize that calprotectin, an inflammation marker, could be a potential predictor of microvascular manifestations in antiphospholipid syndrome. |
What was learned from the study? |
Serum calprotectin levels are increased in antiphospholipid antibody (aPL)-positive patients and could be a potential predictor of microvascular manifestations. |
Serum calprotectin negatively correlated with platelet count (PLT) in aPL-positive patients. |
Physicians should pay more attention to patients with high levels of serum calprotectin, be alert to the occurrence of microvascular manifestations, and adopt appropriate treatment regimens in time. |
Introduction
Antiphospholipid syndrome (APS) is a rare and complicated autoimmune disease characterized by arterial/venous thrombosis and/or recurrent pregnancy morbidity with antiphospholipid antibody (aPL) positivity. Except for thrombotic APS (tAPS) and obstetric APS (oAPS), some aPL-positive patients develop extra-criteria manifestations including thrombocytopenia, hemolytic anemia, and microvascular manifestations. Microvascular manifestations include APS nephropathy, livedo reticularis, valvular lesions, non-stroke central nervous system (CNS) manifestations, myocarditis, catastrophic APS (CAPS), etc. [1,2,3]. Patients with microvascular manifestations have relatively poor prognoses [4, 5]. For those patients, anticoagulation is not enough, and immunosuppressive medications are always needed [6]. Therefore, it is crucial to accurately identify patients who will easily develop microvascular manifestations. However, there has been no generally accepted predictor for microvascular manifestations.
Calprotectin, also known as leukocyte L1 antigen complex, is a heterodimer of S100A8/S100A9 mainly released by neutrophils and monocytes. Extracellular calprotectin is a steady marker of inflammation. Serum and plasma levels of calprotectin are elevated in inflammatory diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and inflammatory bowel disease (IBD) as well as thrombotic diseases such as myocardial infarction and COVID-19 with thrombotic complications [7,8,9]. Studies found that thromboinflammation plays an important role in microvascular manifestations in patients with APS [10]. Ruff et al. [11] indicated that the level of fecal calprotectin in patients with APS is elevated. However, there has been no study focusing on serum calprotectin and its relationship with microvascular manifestations in aPL-positive patients.
In this cohort study, we aimed to explore whether serum calprotectin could be a predictor of microvascular manifestations in aPL-positive patients. These findings will help us identify patients who will easily develop microvascular manifestations and to take appropriate action.
Methods
Study Design
This was a single-center cohort study [12]. Consecutive patients referred to Peking Union Medical College Hospital (PUMCH) from June 2012 to September 2022 with persistent aPLs positivity (detected at least 12 weeks apart) and serum samples were included. Serum samples were obtained from patients during follow-up. Follow-up time was defined as the time between the first occurrence of aPLs positivity and the first occurrence of new-onset microvascular manifestations or the last follow-up visit. Follow-up visits ended in November 2022. Blood samples of age- and sex-matched health controls (HCs) were included. Serum calprotectin was measured using an enzyme-linked immunosorbent assay (ELISA) (Elabscience). The cutoff value was defined as mean + 2 standard deviations (SDs) of HCs.
This study was approved by the Medical Ethics Committee of PUMCH (approval number JS-2038) and carried out in accordance with the Declaration of Helsinki and its later amendments. Written informed consent was obtained from all participants. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of the research.
Data Collection
Blood sampling age, age at the first occurrence of aPLs positivity, gender, smoking history, cardiovascular risk factors, clinical manifestations, and antibody profile of all the patients were collected. Clinical manifestations included thrombosis, pregnancy morbidity, thrombocytopenia, hemolytic anemia, and microvascular manifestations that happened before the last follow-up visit. Microvascular manifestations [1,2,3] included APS nephropathy, livedo reticularis, livedoid vasculopathy lesions, valvular lesions, non-stroke CNS manifestations [13], myocarditis, CAPS [14], pulmonary hemorrhage, multiple bone infarction, retinal microvascular manifestations [15], liver infarction [16], cholecystitis, mesenteric vasculitis, and pancreatitis. APS nephropathy was diagnosed by clinical diagnosis or pathology. Livedo reticularis and livedoid vasculopathy lesion were diagnosed by clinical diagnosis. The valvular lesions were diagnosed by transthoracic echocardiography or transesophageal echocardiography. Non-stroke CNS manifestations were diagnosed by magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) or clinical diagnosis. Myocarditis was diagnosed by MRI. CAPS was diagnosed according to the preliminary classification criteria published in 2003 [1]. Pulmonary hemorrhage was diagnosed by computed tomography. Multiple bone infarction was diagnosed by MRI. Retinal microvascular manifestations were diagnosed by ophthalmological examination and fluorescein angiography. Liver infarction, cholecystitis, mesenteric vasculitis, and pancreatitis were diagnosed by pathology. Laboratory data including blood routine examination, immunoglobulin, complement, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) at the time of blood sampling were collected. The treatment regimen including glucocorticoid, anticoagulation, antiplatelet, hydroxychloroquine, and immunosuppression at the time of blood sampling were collected as well.
Lupus anticoagulation (LA), anticardiolipin antibody (aCL), and anti-β2-glycoprotein I antibody (anti-β2GPI) constituted aPLs. LA was measured according to the recommended three-step procedure with two test systems from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Hemostasis Subcommittee. Activated partial thromboplastin time-based assay (aPTT) and the dilute Russell viper venom time (dRVVT) were used, and aPTT ratio > 1.20 or dRVVT ratio > 1.20 [17] was defined as positivity. aCL and anti-β2GPI were measured by chemiluminescent immunoassay (CLIA) (iFlash CLIA kits provided by YHLO Biotech Co., Shenzhen, China). Medium or high titer of aCL was defined as a titer > 10 U/mL and medium or high titer of anti-β2GPI was defined as a titer > 20 U/mL according to the manufacturer’s instructions. The detection of aCL and anti-β2GPI showed good sensitivity and specificity in our cohort in the previous study [18].
Statistical Analysis
Continuous variables with normally distributed data were presented as means and standard deviations (SD). Medians and interquartile ranges (IQR) (P25, P75) were used for variables with non-normal distribution. Continuous variables were analyzed with Student’s t test or Mann–Whitney U test. Categorical variables were presented as counts and percentages. Categorical variables were analyzed with Pearson chi-square test or Fisher’s exact test. The prognosis was shown by the cumulative events curve. Further comparisons were performed using a log-rank test. The prediction of calprotectin positivity and microvascular manifestations was analyzed by multivariable logistic regression. Variables included in multivariable logistic regression were selected by least absolute shrinkage and selection operator (LASSO) regression analysis. Forest plots were used to illustrate the risk factors. Pearson correlation analysis was used to detect the correlation between serum calprotectin and other laboratory data. p < 0.05 was considered statistically significant.
Results
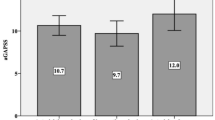
A total of 466 patients were included in the study, among whom 281 (60.3%) could be diagnosed as APS according to the 2006 Sydney Revised Classification Criteria [19]. Among the patients with APS, 217 (77.2%) had primary APS (PAPS) and 64 (22.8%) had secondary APS (SAPS). The mean age was 39.10 ± 13.05 years old, and 359 (77.0%) were female (Table 1). A total of 38 HCs matched for blood sampling age and sex were included. The mean age of HCs was 36.47 ± 5.87 years old, and 30 (78.9%) were female. Patients were followed up for a median of 29.6 months. Serum calprotectin in aPL-positive patients was much higher than that in HCs (649.66 ± 240.79 ng/ml vs 484.62 ± 149.37 ng/ml, p < 0.001). Both the average calprotectin levels in patients with SLE (689.49 ± 269.07 ng/ml) and in patients without SLE (635.85 ± 228.98 mg/ml) were significantly higher than calprotectin in HCs (p < 0.001). In addition, serum calprotectin in patients with microvascular manifestations was much higher than that in patients without microvascular manifestations (693.03 ± 271.90 ng/ml vs 639.43 ± 232.06 ng/ml, p = 0.044) (Fig. 1).
The cutoff value for calprotectin positivity was set as 783.36 ng/ml. Ninety-three patients (20.0%) were positive for serum calprotectin. Calprotectin positivity was significantly associated with SLE (34.4% vs 23.6%, p = 0.033), SAPS (20.4% vs 12.1%, p = 0.036), microvascular manifestations (26.9% vs 17.2%, p = 0.033), non-stroke CNS manifestations (14.0% vs 7.0%, p = 0.029), lupus anticoagulant (LA) positivity (79.6% vs 66.0%, p = 0.011), platelet count (PLT) < 100 × 109/L (20.4% vs 10.5%, p = 0.009), and higher CRP (median [P25, P75], 1.36 [0.55, 3.46] vs 0.97 [0.43, 2.32], p = 0.018) (Table 2). Thrombocytopenia history had no significant relationship with calprotectin positivity (46.2% vs 39.7%, p = 0.250). Among the 89 patients with microvascular manifestations, 25 had positive calprotectin and 64 had negative calprotectin. Among the 377 patients without microvascular manifestations, 68 had positive calprotectin and 309 had negative calprotectin. The specificity of calprotectin as a biomarker of microvascular manifestations was 82% (95% confidence interval [CI] 78–86%).
Fourteen candidate variables (sex, blood sampling age, SLE, microvascular manifestations, arterial thrombosis, venous thrombosis, PLT < 100, hemolytic anemia, LA, aCL, anti-β2GPI, glucocorticoid, hydroxychloroquine, immunosuppression) were included in the LASSO regression analysis to select the variables to predict calprotectin positivity. The lambda.min was 0.0155 and selected seven variables (Supplementary Material Figs. S1, S2). In multivariable logistic regression, microvascular manifestations (odds ratio [OR] 1.90, 95% CI 1.07–3.36) and PLT < 100 (OR 2.04, 95% CI 1.08–3.88) were independently associated with calprotectin positivity in aPL-positive patients (Fig. 2).
During a median follow-up of 29.6 months, there were 53 microvascular manifestations events. The 1-, 3-, and 5-year microvascular manifestation risks were 4.6%, 10.6%, and 17.2%, respectively. Patients with calprotectin positivity tended to develop microvascular manifestations during follow-up (Fig. 3), but the difference was not significant (p = 0.14).
According to the literature review and expert consensus, 12 candidate variables (sex, age at the first occurrence of aPLs positivity, SLE, calprotectin positivity, hypertension, arterial thrombosis, venous thrombosis, thrombocytopenia, hemolytic anemia, LA, aCL, anti-β2GPI) were included in the LASSO regression analysis to select variables to predict microvascular manifestations. The lambda.min was 0.0049 and selected 11 variables (Supplementary Material Figs. S3, S4). In multivariable analysis, age at the first occurrence of aPLs positivity (OR 0.98, 95% CI 0.96–1), SLE (OR 2.08, 95% CI 1.15–3.75), calprotectin positivity (OR 1.83, 95% CI 1.02–3.26), hypertension (OR 2.73, 95% CI 1.36–5.45), hemolytic anemia (OR 2.66, 95% CI 1.13–6.23), and anti-β2GPI antibody (OR 2.06, 95% CI 1.11–3.83) could independently predict microvascular manifestations in aPL-positive patients (Fig. 4).
Pearson correlation analysis showed that serum calprotectin had positive correlations with ESR (R = 0.155, p = 0.001), CRP (R = 0.119, p = 0.014), and a negative correlation with PLT (R = − 0.101, p = 0.031) at the time of blood sampling (Fig. 5).
Discussion
This is the first study showing that calprotectin might be a potential predictor of microvascular manifestations in aPL-positive patients based on a large cohort. Serum calprotectin in aPL-positive patients was much higher than that in HCs (649.66 ± 240.79 ng/ml vs 484.62 ± 149.37 ng/ml, p < 0.001). Age of the first occurrence of aPLs positivity (OR 0.98, 95% CI 0.96–1), SLE (OR 2.08, 95% CI 1.15–3.75), calprotectin positivity (OR 1.83, 95% CI 1.02–3.26), hypertension (OR 2.73, 95% CI 1.36–5.45), hemolytic anemia (OR 2.66, 95% CI 1.13–6.23), and anti-β2GPI antibody (OR 2.06, 95% CI 1.11–3.83) are independent risk factors for microvascular manifestations in aPL-positive patients. Serum calprotectin had positive correlations with ESR (R = 0.155, p = 0.001), CRP (R = 0.119, p = 0.014), and a negative correlation with PLT (R = − 0.101, p = 0.031) at the time of blood sampling.
Extra-criteria manifestations, including microvascular manifestations, reflect a subtype of patients with aPLs positivity [4]. Those patients have a higher prevalence of arterial thrombosis, preeclampsia, relapse, and triple aPL positivity [5]. In the new APS classification criteria, microvascular manifestations were listed as clinical criteria as well, suggesting that microvascular manifestations play an important role in patients with APS [2]. However, there has been no generally accepted serum predictor for microvascular manifestations.
Calprotectin is a heterodimer of S100A8/S100A9 belonging to the family of Ca2+ binding proteins. It is mainly secreted by neutrophils and monocytes by necrosis, neutrophil extracellular trap formation, and Golgi-independent alternative pathway [20,21,22]. Macrophages, platelets, epithelial cells, keratinocytes, and cancer cells can also express calprotectin. Extracellular calprotectin is a steady marker of inflammation. It induces a proinflammatory response on endothelial cells, monocytes, neutrophils, and T cells mainly by Toll-like receptor 4 (TLR4) and receptor of advanced glycation end products (RAGE) [7]. Serum and plasma levels of calprotectin are elevated in many inflammatory diseases and thrombotic diseases [7]. Serum calprotectin is significantly higher in patients with SLE and has a positive correlation with disease activity [23]. In patients with COVID-19, calprotectin in circulation is correlated with thrombosis and poor prognosis [9, 24]. In patients with IBD, fecal calprotectin can be used to distinguish IBD from irritable bowel syndrome and monitor disease activity [25].
In our study, we found that serum calprotectin levels in aPL-positive patients were much higher than that in HCs regardless of SLE. There has been no study focusing on serum calprotectin levels in patients with APS before. Ruff et al. [11] found that the level of fecal calprotectin in patients with APS is elevated. Lood et al. [26] indicated that platelet calprotectin levels were increased in patients with SLE, particularly in patients with aPL positivity. However, the platelet level of calprotectin had no relationship with serum or plasma levels. These suggest that there might be an increment in both the expression and release of calprotectin in aPL-positive patients.
As an inflammation marker, calprotectin had positive correlations with ESR and CRP in our study. Patients with calprotectin positivity had a higher prevalence of microvascular manifestations. Endothelial cell dysfunction plays an important role in the pathogenesis of microvascular manifestations in patients with APS. Antibodies from patients with APS stimulate the mammalian target of rapamycin complex (mTORC) in endothelial cells, which is involved in the pathogenesis of microvascular manifestations [27]. Agostinis et al. [28] found that proinflammatory factors were needed for the binding between β2GPI and endothelial cells. These might explain the reason for the correlation between calprotectin and microvascular manifestations. Calprotectin could be an independent risk factor for microvascular manifestations, suggesting that patients in a hyperinflammation state are more likely to have endothelial cell dysfunction and develop microvascular manifestations.
Thrombocytopenia occurs in 20–50% of aPL-positive patients [29]. There are many proposed mechanisms of thrombocytopenia in patients with APS [30]. Anti-β2GPI–β2GPI complex could activate platelets by binding the glycoprotein Ibα (GPIbα) receptor, which might lead to thrombocytopenia [31]. In this study, we found that calprotectin was associated with thrombocytopenia at the time of blood sampling, but not thrombocytopenia history, which indicated that the process of calprotectin release was associated with the decrease of PLT. In vitro, calprotectin activated platelets with GPIbα as the receptor and the supporting role of CD36. Glycoprotein IIb/IIIa (GPIIb/IIIa) was activated, the expression of p-selectin was upregulated, and platelet-neutrophil aggregates were increased, but there was no platelet aggregation, which suggested a new mechanism of platelet activation [24]. Therefore, calprotectin might lead to thrombocytopenia by binding GPIbα and activating platelets.
Except for serum calprotectin, age at the first occurrence of aPLs positivity, SLE, hypertension, hemolytic anemia, and anti-β2GPI antibody were also found to be independent risk factors for microvascular manifestations, which was similar to the results in other studies. Anti-β2GPI antibody, double aPL positivity, cerebrovascular events history, and hypertension were more prevalent in patients with PAPS and extra-criteria manifestations [32]. Huang et al. [33] found that SLE, anti-β2GPI antibody, LA, and triple aPL positivity were associated with extra-criteria manifestations in aPL-positive patients. Thrombocytopenia, elevated CRP, and anti-β2GPI antibody were found to be independent risk factors for microvascular manifestations in patients with PAPS [34]. The reason for anti-β2GPI antibody contributing to microvascular manifestations remains unclear. By binding to β2GPI, the antibody activates endothelial cells, which leads to inflammation, endothelial cell proliferation, and intimal hyperplasia [35]. This process might result in microvascular manifestations.
This study has several limitations. Firstly, the results should be validated in another APS cohort to confirm the relation between calprotectin and microvascular manifestations. Secondly, a single test of calprotectin could not reflect the whole picture of the disease for patients. Studies monitoring serum calprotectin levels continuously are needed to find out a more specific relation between calprotectin and disease progression. Thirdly, this study did not elucidate the mechanism that enables calprotectin to cause microvascular manifestations and thrombocytopenia. Cell and mouse experiments are needed to explore the pathogenesis of calprotectin. Fourthly, the treatment regimen had an impact on the occurrence of microvascular manifestations, but the influence could not be eliminated when we predict microvascular manifestations.
Conclusions
Serum calprotectin levels are increased in aPL-positive patients and could be a potential predictor of microvascular manifestations in patients with antiphospholipid syndrome. Physicians should pay more attention to patients with high levels of serum calprotectin, be alert to the occurrence of microvascular manifestations, and adopt appropriate treatment regimens in a timely manner.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Asherson RA, Cervera R, de Groot PG, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12(7):530–4.
Barbhaiya M, Zuily S, Naden R, et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Arthritis Rheumatol. 2023;75:1687–702.
Sayar Z, Moll R, Isenberg D, Cohen H. Thrombotic antiphospholipid syndrome: a practical guide to diagnosis and management. Thromb Res. 2021;198:213–21.
Qi W, Zhao J, Huang C, et al. Clinical characteristics and prognosis of patients with antiphospholipid antibodies based on cluster analysis: an 8-year cohort study. Arthritis Res Ther. 2022;24(1):140.
Guedon AF, Catano J, Ricard L, et al. Non-criteria manifestations in primary antiphospholipid syndrome: a French multicenter retrospective cohort study. Arthritis Res Ther. 2022;24(1):33.
Erkan D. Expert perspective: management of microvascular and catastrophic antiphospholipid syndrome. Arthritis Rheumatol. 2021;73(10):1780–90.
Pruenster M, Vogl T, Roth J, Sperandio M. S100A8/A9: from basic science to clinical application. Pharmacol Ther. 2016;167:120–31.
Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013. https://doi.org/10.1155/2013/828354.
Zuo Y, Zuo M, Yalavarthi S, et al. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis. 2021;51(2):446–53.
Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906–18.
Ruff WE, Dehner C, Kim WJ, et al. Pathogenic autoreactive T and B cells cross-react with mimotopes expressed by a common human gut commensal to trigger autoimmunity. Cell Host Microbe. 2019;26(1):100–113.e8.
Huang C, Zhao J, Tian X, Wang Q, Xu D, Li M, Zeng X. RheumCloud App: A novel mobile application for the management of rheumatic diseases patients in China. Rheumatol. Immunol. Res. 2022;3(4):184–89. https://doi.org/10.2478/rir-2022-0033
You H, Zhao J, Li M, Zeng X. Recurrent non-stroke central neurologic manifestations in primary antiphospholipid syndrome. Rheumatol Immunol Res. 2022;3(2):93–4.
Huang C, Zhao Y, Tian X, et al. Early recognition of catastrophic antiphospholipid syndrome in patients with antiphospholipid syndrome based on a Chinese cohort study. Clin Exp Rheumatol. 2023;41(5):1017–23.
Xie Z, Li H, Qi W, et al. Characteristics and risk factors of retinal vasculopathy in antiphospholipid syndrome. Lupus. 2022;31(2):178–86.
Li C, Zhao J, Zhao Y. Hepatic infarction caused by antiphospholipid syndrome secondary to systemic lupus erythematosus. J Rheumatol. 2019;46(7):755–6.
Devreese KMJ, de Groot PG, de Laat B, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. 2020;18(11):2828–39.
Hu C, Li S, Xie Z, et al. Comparison of different test systems for the detection of antiphospholipid antibodies in a Chinese cohort. Front Immunol. 2021;12: 648881.
Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295–306.
Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272(14):9496–502.
Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639.
Voganatsi A, Panyutich A, Miyasaki KT, Murthy RK. Mechanism of extracellular release of human neutrophil calprotectin complex. J Leukoc Biol. 2001;70(1):130–4.
Soyfoo MS, Roth J, Vogl T, Pochet R, Decaux G. Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. J Rheumatol. 2009;36(10):2190–4.
Colicchia M, Schrottmaier WC, Perrella G, et al. S100A8/A9 drives the formation of procoagulant platelets through GPIbalpha. Blood. 2022;140(24):2626–43.
Tibble JA, Bjarnason I. Non-invasive investigation of inflammatory bowel disease. World J Gastroenterol. 2001;7(4):460–5.
Lood C, Tyden H, Gullstrand B, et al. Platelet-derived S100A8/A9 and cardiovascular disease in systemic lupus erythematosus. Arthritis Rheumatol. 2016;68(8):1970–80.
Canaud G, Bienaime F, Tabarin F, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014;371(4):303–12.
Agostinis C, Biffi S, Garrovo C, et al. In vivo distribution of beta2 glycoprotein I under various pathophysiologic conditions. Blood. 2011;118(15):4231–8.
Artim-Esen B, Diz-Kucukkaya R, Inanc M. The significance and management of thrombocytopenia in antiphospholipid syndrome. Curr Rheumatol Rep. 2015;17(3):14.
El Hasbani G, Saliba AN, Uthman I, Taher AT. Hematological manifestations of antiphospholipid syndrome: going beyond thrombosis. Blood Rev. 2023;58:101015.
Shi T, Giannakopoulos B, Yan X, et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54(8):2558–67.
Radin M, Ugolini-Lopes MR, Sciascia S, Andrade D. Extra-criteria manifestations of antiphospholipid syndrome: risk assessment and management. Semin Arthritis Rheum. 2018;48(1):117–20.
Huang C, Hu C, Zhao J, et al. Extra-criteria clinical manifestations of antiphospholipid antibody should not be ignored. Chin Med J (Engl). 2022;135(18):2251–2.
Sun Y, Zhao J, Zhang P, et al. Clinical characteristics and risk factors of microvascular involvement in primary antiphospholipid syndrome: a longitudinal single-center study in China. Lupus. 2019;28(13):1558–65.
Tektonidou MG. Cardiovascular disease risk in antiphospholipid syndrome: thrombo-inflammation and atherothrombosis. J Autoimmun. 2022;128:102813.
Acknowledgements
We thank the participants of the study.
Funding
This work was supported by the Chinese National Key Technology R&D Program, Ministry of Science and Technology [2021YFC2501305 to JL.Z.]; Beijing Municipal Science & Technology Commission [Z201100005520027 to XF.Z.]; Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS) [2021-I2M-1-005 to XF.Z.]; and National High Level Hospital Clinical Research Funding [2022-PUMCH-B-013 to XF.Z., 2022-PUMCH-A-008 to JL.Z.].
Author information
Authors and Affiliations
Contributions
Yuan Zhao designed the study, detected data, analyzed data, interpreted results, and wrote the manuscript; Wanting Qi designed the study and detected data; Can Huang and Yangzhong Zhou interpreted results and revised the manuscript; Qian Wang, Xinping Tian, Mengtao Li, Yan Zhao, and Xiaofeng Zeng supervised the study and revised the manuscript; Jiuliang Zhao designed the study, interpreted results, and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Yuan Zhao, Wanting Qi, Can Huang, Yangzhong Zhou, Qian Wang, Xinping Tian, Mengtao Li, Yan Zhao, Xiaofeng Zeng and Jiuliang Zhao have nothing to disclose.
Ethical Approval
This study was approved by the Medical Ethics Committee of Peking Union Medical College Hospital (approval number: JS-2038) and carried out in accordance with the Declaration of Helsinki and its later amendments. Written informed consent was obtained from all patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhao, Y., Qi, W., Huang, C. et al. Serum Calprotectin as a Potential Predictor of Microvascular Manifestations in Patients with Antiphospholipid Syndrome. Rheumatol Ther 10, 1769–1783 (2023). https://doi.org/10.1007/s40744-023-00610-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00610-9