Abstract
Introduction
Patients with connective tissue disorders (CTD) and pulmonary arterial hypertension (PAH) have a poorer prognosis than those with other PAH etiologies. This study assessed the impact of CTD on healthcare outcomes among PAH patients with and without CTD comorbidities that were treated with oral selexipag.
Methods
The study utilized Optum’s de-identified Clinformatics® Data Mart Database (2007–2021) from January 1, 2014 to June 30, 2019, and identified patients with PAH without CTD and PAH with CTD treated with oral selexipag. Patients had ≥ 12-month baseline period with no requirement for a minimum follow-up period. Patients were followed until any of the following events: discontinuation of oral selexipag, or health plan disenrollment, or death, or presence of a diagnosis claim for CTEPH, or study end date, whichever occurred first. PAH-related hospitalizations, PAH disease progression, and healthcare utilizations and costs were assessed in the follow-up period. The Cox proportional hazards model was used to evaluate the time to hospitalization and generalized linear models were used to examine healthcare costs and utilization between the two cohorts.
Results
In the analysis, 237 PAH without CTD, and 80 PAH patients with CTD comorbidities prescribed oral selexipag were included. The PAH without CTD comorbidities cohort was older (65 vs. 63 years old), had proportionately less females (72 vs. 83%), and higher comorbidity burden than PAH with CTD comorbidities (mean CCI index 3 vs. 2). After adjusting for potential confounders, the risk for PAH-related hospitalization (hazard ratio (HR) 1.13, p value 0.641), all-cause hospitalization (HR 1.09, p value: 0.765), and PAH disease progression (HR 1.14, p value 0.522) between the two cohorts were similar. After adjusting for baseline demographic and clinical characteristics, PAH with CTD comorbidities incurred higher total mean all-cause PAH-related medical care costs compared to PAH without CTD comorbidities.
Conclusions
In this real-world study, the risk of hospitalization and PAH disease progression were similar between the two cohorts who received oral selexipag. The results from this study corroborate findings of the GRIPHON post hoc analysis of PAH-associated CTD patients and support oral selexipag use in PAH-CTD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
A knowledge gap exists regarding the impact of connective tissue disorders (CTD) comorbidities on pulmonary arterial hypertension (PAH) clinical outcomes among oral selexipag users in the US. |
There is limited information regarding the clinical outcomes associated with oral selexipag use among CTD-associated PAH in real-world clinical practice. |
What was learned from this study? |
This real-world study shows that the risk of clinical outcomes such as all-cause hospitalization, PAH hospitalization, and PAH disease progression were not statistically significantly different for CTD-associated PAH patients compared to those with PAH not associated with CTD comorbidities who were prescribed oral selexipag after adjusting for potential measured confounders. |
Oral selexipag therapy provides similar clinical benefits for patients with PAH. |
Introduction
Pulmonary arterial hypertension (PAH) is a severe and progressive disease associated with progressive loss and obstruction of the pulmonary vascular bed, leading to elevated pulmonary arterial pressure which can eventually lead to right heart failure and death [1,2,3]. Connective tissue disorders (CTDs; such as systemic sclerosis [SSc, or scleroderma], mixed connective tissue disease, and systemic lupus erythematosus) are the second leading cause of PAH after idiopathic PAH (IPAH), with CTD-associated PAH representing up to 13% of PAH [4]. Among the CTDs, SSc has the highest prevalence in PAH, and has historically been associated with poor PAH prognosis [3, 5]. The REVEAL data (the Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management) showed that CTD-associated PAH patients had worse survival and higher number of hospitalizations than IPAH patients [4]. In addition, a pool analysis of 11 phase III randomized controlled trials (RCT) of PAH therapies showed treatment is less effective in patients with CTD-associated PAH compared with patients with IPAH [6]. However, since the publication of this pool analysis, new novel therapies have emerged for PAH such as oral selexipag.
Oral selexipag is a selective prostacyclin IP receptor agonist and was notably studied in the GRIPHON trial that included 1156 PAH patients, the majority of whom had IPAH (649 patients) or CTD-associated PAH (334 patients). In the overall study population, oral selexipag reduced the risk for morbidity and mortality by 40% compared with placebo [7]. In a post hoc analysis of the GRIPHON trial, oral selexipag reduced the risk of morbidity and mortality by 41% compared with placebo among CTD-associated PAH patients. This treatment effect of oral selexipag among CTD-associated PAH is consistent with the overall GRIPHON population and in patients with IPAH/heritable PAH [8].
Although the study results of the GRIPHON trial data suggest that CTD-associated PAH patients respond well to oral selexipag, there is limited information regarding the clinical outcomes associated with oral selexipag use among PAH patients with CTD comorbidities in real-world clinical practice. A knowledge gap exists regarding the impact of CTD comorbidities on PAH clinical outcomes among oral selexipag users in the US. Using real-world data, this study evaluated the impact of oral selexipag on clinical outcomes, including hospitalization, PAH disease progression, health care utilization and medical costs, in PAH patients with and without CTD comorbidities.
Methods
Study Design and Data Sources
The study utilized the Optum de-identified Clinformatics® Mart Database with comprehensive demographic, clinical, and treatment information identified using International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM, ICD-10-CM) codes, National Drug Codes (NDCs), and Healthcare Common Procedure Coding System (HCPCS) codes. The analyses did not involve the collection, use, or transmittal of individual identifiable data. Thus, institutional review board approval to conduct this study was not required.
Study Population
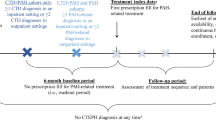
This retrospective cohort study included patients ≥ 18 years of age identified between December 1, 2015, and December 31, 2019. Patients were required to have ≥ 1 inpatient or emergency room or ≥ 2 outpatient pulmonary hypertension (PH)/PAH diagnosis claims (ICD-9-CM: 416.0, 416.8 or ICD-10-CM: I27.0, I27.2, I27.89). The first observed PH/PAH diagnosis claim date was defined as the first observed diagnosis date. Patients were also required to have ≥ 1 claim for oral selexipag on or after the first observed diagnosis date and the first oral selexipag claim date during the identification period was defined as the index date. Continuous health plan enrollment was required for ≥ 12 months prior to the index date (baseline period). Patients who had oral selexipag, other prostacyclin pathway agents (treprostinil, epoprostenol, and iloprost), chronic thrombo-embolic pulmonary hypertension (CTEPH, ICD-9-CM: not available; ICD-10-CM: I27.24), lung transplant or balloon atrial septostomy during the baseline period were excluded from the study.
PAH/PH patients were stratified into PAH with and without CTD comorbidities cohorts. Additionally, PAH patients with CTD comorbidities were required to have ≥ 1 diagnosis claim for any of the following CTD conditions at any time during the baseline period (systemic sclerosis [SSc] ICD-9-CM: 710.1; ICD-10-CM: M34; systemic lupus erythematosus [SLE] ICD-9-CM: 517.8, 710.0 or ICD-10-CM: M32; Sicca syndrome: ICD-9-CM: 517.8, 710.2 or ICD-10-CM: M35.0; dermatomyositis or polymyositis: ICD-9-CM: 710.3, 710.4 or ICD-10-CM: M33; mixed CTD: ICD-9-CM: 710.8, 710.9 or ICD-10-CM: M35.1 or M36.8).
Baseline Characteristics
Patient demographic characteristics were evaluated as of the index date and included sex, age, and geographic region. Clinical characteristics were evaluated during the baseline period and included Deyo-Charlson Comorbidity Index (CCI) scores [9, 10] and individual comorbidities which were identified using ICD-9/10-CM codes (Supplementary Material Figure S2; individual ICD 9/10 codes for baseline comorbidities can be found in Supplementary Material Table S2). All-cause health care utilization and costs during the baseline period were also evaluated.
Study Outcomes
Time to first all-cause hospitalization and time to first PAH-related hospitalization after the index date were examined and reported as the primary outcomes. The time to PAH disease progression was determined using composite endpoints and was defined as time from the index date to earliest of any of the following during the follow-up period:
-
Initiation of parenteral prostanoids treatment was defined as the date of the first prescription of intravenous (IV)/subcutaneous (SC) prostanoids (e.g., epoprostenol, treprostinil)
-
Date of all-cause death,
-
Lung transplant claim date,
-
Balloon atrial septostomy claim date,
-
PAH-related hospitalization claim date, OR
-
PAH-related ER visit claim date.
The healthcare resource utilization (HCRU) and costs were examined during the entire follow-up period for patients and included all-cause and PAH-related utilization and associated costs. Costs, defined as standardized gross payments, were reported as per patient per month (PPPM) and adjusted to 2019 US dollars using the medical care component of the Consumer Price Index (CPI) [11].
Statistical Analysis
All variables were analyzed descriptively with means, standard deviations for continuous variables and counts, percentages for categorical variables. p values were calculated using chi-square tests for categorical and Student’s t tests for continuous variables. Demographics (age, sex, geographic region) and clinical characteristics (CCI scores, individual comorbidities) were explored during the baseline period. Time to PAH-related hospitalization, time to all-cause hospitalization and time to PAH disease progression were compared between PAH patients with and without CTD comorbidities cohorts using Kaplan–Meier analyses with log-rank tests and Cox proportional hazards models that reported the hazard ratio with the 95% confidence interval. Generalized linear models (GLMs) were used to evaluate the HCRU and costs during the follow-up period. The Cox proportional hazards and the GLMs were adjusted for statistically significant baseline demographic and clinical characteristics including age, sex, geographic region, baseline oral selexipag regimen, apnea, obesity, and interstitial lung disease. Analyses were performed using SAS® for Windows, Version 9.4 (SAS Institute, Cary, NC, USA). Adjustment for multiple testing was not performed.
Compliance with Ethical Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Since this study did not involve the collection, use, or transmittal of individually identifiable data, it was deemed exempt from Institutional Review Board review by Solutions IRB. Both the datasets and the security of the offices where analysis was completed (and where the datasets are kept) meet the requirements of the Health Insurance Portability and Accountability Act of 1996. Solutions IRB determined this study to be EXEMPT from the Office for Human Research Protections (OHRP)’s Regulations for the Protection of Human Subjects (45 CFR 46) under Exemption 4: Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. The HIPAA Authorization Waiver was granted in accordance with the specifications of 45 CFR 164.512(i). This project was conducted in full accordance with all applicable laws and regulations, and adhered to the project plan that was reviewed by Solutions Institutional Review Board.
Results
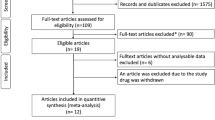
A total of 317 patients were identified who initiated oral selexipag, among which 74.8% (n = 237) were identified as PAH patients without CTD comorbidities and 15.2% (n = 80) were PAH patients with CTD comorbidities. (Supplementary Material Figure S1).
Baseline Characteristics
Among the PAH patients with and without CTD comorbidities cohorts, respectively, patients had mean ages of 62.7 and 64.8 years (p = 0.221), were predominately female (82.5% and 72.2%; p = 0.065), and mostly resided in the Southern US region (47.5% and 53.2%; p = 0.381). Mean CCI scores were lower among PAH patients with CTD comorbidities when compared to PAH patients without CTD comorbidities (2.3 and 3.1 respectively; p = 0.013). The most common comorbidities among the PAH with CTD comorbidities cohort were hypertension (91.3%) and coronary artery disease (42.5%), followed by interstitial lung disease (40.0%). The most common comorbidities among PAH without CTD comorbidities cohort were hypertension (84.4%) and chronic obstructive pulmonary disease (55.7%), followed by apnea (48.5%) during the 12-month baseline period. PAH without CTD comorbidities cohort had higher proportion of patients with ≥ 1 inpatient visit during the baseline period (PAH with CTD comorbidities = 49.8% vs. PAH without CTD comorbidities = 58.8%) and longer length of stay for all inpatient visits (PAH with CTD comorbidities = 0.58 days vs. PAH without CTD comorbidities = 0.71 days). The number of outpatient visits were 5.84 and 4.62 days in the PAH patients with and without CTD comorbidities cohorts, respectively. PAH patients with CTD comorbidities had higher average total all-cause health care costs as compared to PAH without CTD comorbidities during the 12-month baseline period ($7719 vs. $6078, respectively) (Table 1).
Unadjusted Results
Time to All-cause and PAH-Related Hospitalization
PAH patients with CTD comorbidities had a mean follow-up time of 231 days compared to 224 days for PAH patients without CTD comorbidities (p = 0.858). Among PAH patients with CTD comorbidities, 29 (36.3%) and 26 (32.5%) had all-cause and PAH-related hospitalization, respectively. The median time to all-cause and PAH-related hospitalization was observed to be 450 and 484 days, respectively, among PAH patients with CTD comorbidities. Similarly, among the PAH patients without CTD comorbidities, 67 (28.3%) and 26 (26.2%) patients had all-cause and PAH-related hospitalization, respectively. The median time to all-cause and PAH-related hospitalization was observed to be 495 and 503 days, respectively, among PAH patients without CTD comorbidities. However, no statistically significant difference was observed in time to all-cause and time to PAH-related hospitalization between PAH patients with CTD comorbidities treated with oral selexipag and PAH patients without CTD comorbidities treated with oral selexipag (Table 2).
Time to PAH Disease Progression
PAH with CTD comorbidities cohort had a similar proportion of patients with PAH disease progression during the follow-up period compared to the PAH patients without CTD comorbidities (CTD-associated PAH = 56.3% vs. PAH not associated with CTD = 47.3%; p = 0.164). PAH-related hospitalization and PAH-related ER visits accounted for 86.6% of the events in the PAH disease progression composite endpoint.
Time to PAH disease progression was observed to be similar among PAH patients with CTD comorbidities when compared to PAH patients without CTD comorbidities (CTD-associated PAH = 290 days vs. PAH patients not associated with CTD = 329 days; p = 0.744) (Table 2).
HCRU and Costs During the Follow-up Period
The unadjusted total all-cause and PAH-related medical healthcare PPPM costs were similar between the PAH patients with and without CTD comorbidities cohorts. The outpatient office all-cause healthcare PPPM costs were higher (CTD-associated PAH $210 vs. PAH patients not associated with CTD = $157; p = 0.013) for the PAH with CTD comorbidities cohort when compared to the PAH without CTD comorbidities cohort (Table 2).
Multivariable Results
PAH-Related Hospitalization and Costs
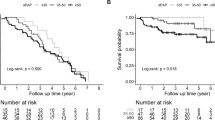
The PAH with CTD cohort had a similar risk of PAH-related hospitalization during the follow-up period compared to the PAH without CTD comorbidities after adjusting for potential measured confounders [hazard ratio (HR) = 1.13; 95% CI = 0.67–1.90, p value = 0.6410] (Fig. 1).
After adjusting for the baseline demographic and clinical characteristics there were no statistically significant differences observed in total PAH-related medical costs between PAH patients with and without CTD comorbidities cohorts (PAH with CTD comorbidities = $2568 PPPM vs. PAH without CTD comorbidities = $2059 PPPM; p = 0.157). The total all-cause medical costs (inpatient + outpatient) were significantly higher among the PAH with CTD comorbidities when compared to PAH without CTD comorbidities cohort (PAH with CTD comorbidities = $6312 PPPM vs. PAH without CTD comorbidities CTD = $4663 PPPM, p value = 0.047). PAH with CTD comorbidities cohort had higher inpatient and outpatient cost than PAH without CTD comorbidities cohort with all-cause ER cost being a significant driver for the PAH with CTD comorbidities cohort (PAH with CTD comorbidities $406 PPPM vs. PAH without CTD comorbidities = $253 PPPM, p = 0.004) (Supplementary Material Table S1).
All-Cause Hospitalization
The PAH with CTD comorbidities cohort had a similar risk of all-cause hospitalization during the follow-up period compared to the PAH patients without CTD comorbidities after adjusting to potential measured confounders [hazard ratio (HR) = 1.09; 95% CI = 0.71–1.28, p value = 0.7650] (Fig. 1).
Disease Progression
The PAH with CTD comorbidities cohort had similar risk of PAH disease progression during the follow-up period compared to the PAH patients without (Fig. 2) CTD comorbidities after adjusting for potential measured confounders [hazard ratio (HR) = 1.14; 95% CI = 0.76–1.75, p value = 0.5220] (Fig. 3).
Discussion
This study examined hospitalization rate, PAH disease progression, medical costs, and healthcare utilization among PAH patients with and without CTD comorbidities who were prescribed oral selexipag. Historically, PAH treatment appeared less effective in CTD-associated PAH patients compared to IPAH patients [3, 6, 12]. However, the results from this study suggested that risks of all-cause hospitalization, PAH hospitalization, or PAH disease progression, were not statistically significantly different between PAH patients with and without CTD comorbidities among oral selexipag users. The results of this study complement the data that were observed in the GRIPHON post-hoc analysis that showed the treatment effects of oral selexipag for morbidity and mortality were consistent between CTD-associated patients, IPAH patients, and the overall study population. Further, the GRIPHON post hoc analysis looked at CTD-associated PAH based on the CTD subtype specifically patients with PAH-SSc and PAH-SLE were examined. That post hoc analysis showed that despite the differences in CTD subtypes, the treatment benefits such as reducing risk of PAH disease progression and hospitalization by oral selexipag utilization was consistent for the CTD subtypes and other PAH patients without CTD [6, 13]. However, the current study differed from the GRIPHON post hoc analysis in the groups that were compared. In this study, two groups of oral selexipag users were compared since a control group without oral selexipag is assumed to have less severe disease leading to introduction of selection bias (Table 3).
There are not many other studies that have investigated the impact of PAH medication on health outcomes among CTD-associated PAH. Rhee et al. conducted a meta-analysis of 11 RCTs to compare the effect of PAH therapy between IPAH and CTD-associated PAH. They found the effect of PAH treatment was less effective in improving 6-min walk distance and preventing clinical worsening in CTD-associated PAH patients compared to IPAH. However, several trials that were included in this meta-analysis had patients that did not receive background PAH therapies. Since the publication of this meta-analysis, new novel therapies such as oral prostacyclin pathway agents were introduced in the treatment landscape and combination therapy is now regarded as the standard of care in PAH. A recent meta-analysis of 11 RCTs and 19 registries found a similar risk reduction in morbidity and mortality between CTD-associated PAH and the overall PAH population, suggesting a potential benefit from currently available PAH therapies. This meta-analysis included trials of modern therapy to date as well as the more frequent use of combination therapy. In our study, about 86.25% of CTD-associated PAH patients were on combination therapy at baseline. Previous studies have indicated that combination therapy is effective in CTD-associated PAH patients [14, 15]. However, in this present study, we cannot determine the magnitude to which combination therapy helped improved the study outcomes in CTD-associated PAH patients.
In this study, PAH-related hospitalization and PAH disease progression were the primary and secondary outcomes, respectively. These selected outcomes are clinically important to PAH patients because PAH disease progression or hospitalization due to PAH events are potential risk factors for mortality [16]. Unlike the 6-min walk test outcome that was used in Rhee et al. meta-analysis to assess PAH treatment response, the composite PAH disease progression outcome that was measured in this study is potentially less influenced by non-cardiopulmonary manifestations of CTD [6].
In addition to examining clinical outcomes such as hospitalization and PAH disease progression, this study also examined the cost and utilization associated with PAH patients with or without CTD comorbidities who were prescribed oral selexipag. Adjusted all-cause healthcare utilization and PAH-related utilization were similar between the two cohorts; however, PAH patients with CTD were observed to have higher all-cause ER PPPM costs and higher mean total medical PPPM costs compared to PAH patients without CTD comorbidities. One possible explanation for the observed PPPM cost difference, in part, is due to the additional comorbidities that are prevalent in patients with CTD. In our study, PAH patients with CTD comorbidities had approximately three times the proportion of patients with interstitial lung disease compared to PAH patients without CTD comorbidities and may affect the all-cause healthcare PPPM costs. PAH-related costs for PAH patients with CTD comorbidities were higher than PAH patients without CTD comorbidities, but the difference was not statistically significant.
The strength of the study comes from the administrative claims database, which covers multiple providers and physician visits well recorded in claims data as patient consent is not necessary for collecting administrative claims data.
A limitation of the study is the lack of specificity of ICD codes in the World Health Organization PAH clinical classification and PAH etiology. Furthermore, there are no specific ICD codes for PAH and therefore we require all patients in the study to receive at least one PAH medication to increase the specificity of the patient selection for PAH.
Replication of this study utilizing EMR data or hospital derived data, which may include clinical data points such as right heart catheterization, echocardiogram, and hemodynamic results, and radiologic patterns for PAH diagnosis and progression, would further validate findings of this study.
It is also important to note that the ICD-9 codes do not distinguish PAH from CTEPH and therefore patients with CTEPH diagnosis in the baseline were excluded and CTEPH diagnosis in the follow-up was used as a censoring event. However, the impact would be minimal given the time period contributing to patient identification prior to ICD CM code change was short.
Another limitation of the study pertains to those observed in the claims data. The presence of a diagnosis code on a medical claim is not a positive presence of disease. The presence of a claim for a filled prescription does not indicate whether the medication was consumed or that it was taken as prescribed. The lack of completeness of the mortality data in the Optum Clinformatics® DoD database may lead to underrepresentation of the death data in the current study. The clinical and disease-specific parameters not captured in claims data could influence the study outcomes, for e.g., severity of the patients which cannot be determined in the claims data.
The definition of PAH disease progression may vary across different studies, which may result in conflicting information on PAH disease progression. The definition of disease progression used in this study could only capture serious changes in PAH disease progression so results should be interpreted with this consideration. Also, the Optum Clinformatics® database reports a ‘standardized cost’ figure that is related to allowable charges and should not be interpreted as the cost of services or medications. It might have under-represented the costs for commercially insured patients and conversely overestimated costs for Medicare patients with supplemental coverage.
This study did not consider undifferentiated CTD, which could have introduced misclassification of PAH patients in some cases. However, this is usually a small proportion and therefore the impact would be minimal. Further, this study did not examine CTD subtypes and CTD medication use due to small sample size of PAH with CTD cohort. Future studies with sufficient sample could adjust for severity of PAH with CTD patients by CTD subtypes and CTD-related medication use.
This study reported and adjusted for PAH therapies at baseline but did not investigate CTD medications. Such study could provide insight into PAH patients with CTD but since the objective of the study was to compare PAH patients with CTD and PAH patients without CTD this limitation would have had a minimal impact.
Due to small sample size, the analysis may be underpowered to detect small differences between PAH with and without CTD. Interpretation of the results should be made with some caution, especially because adjustment for multiple testing was not performed. Finally, the comparability between the two groups might have been affected due to the discrepancy in the sample size of the groups.
Conclusions
In this real-world study, the risk of hospitalization and PAH disease progression were similar among PAH patients with and without CTD comorbidities who received oral selexipag. The results from this study corroborate the findings of the GRIPHON post hoc analysis of PAH-associated CTD patients and the benefit associated with selexipag use in PAH-CTD patients.
References
Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493–537.
Montani D, Gunther S, Dorfmuller P, et al. Pulmonary arterial hypertension. Orphanet J Rare Dis. 2013;8(97):6.
Mandras SA, Mehta HS, Vaidya A. Pulmonary hypertension: a brief guide for clinicians. Mayo Clin Proc. 2020;95:1978–88.
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138(6):1383–94. https://doi.org/10.1378/chest.10-0260.
Rubenfire M. Guidelines for diagnosis and treatment of pulmonary hypertension. Am Coll of Cardiology. 2015. https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2015/...5/15/19/2015-esc-ers-guidelines-for-the-diagnosis-and-treatment-of-ph Accessed 8 Feb 2021.
Rhee RL, Gabler NB, Sangani S, Praestgaard A, Merkel PA, Kawut SM. Comparison of treatment response in idiopathic and connective tissue disease-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192(9):1111–7. https://doi.org/10.1164/rccm.201507-1456OC.
Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–33. https://doi.org/10.1056/NEJMoa1503184.
Gaine S, Chin K, Coghlan G, et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1602493. https://doi.org/10.1183/13993003.02493-2016.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum, 2012, Paper 335–2012.
Dufour R, Pruett J, Hu N, et al. Healthcare resource utilization and costs for patients with pulmonary arterial hypertension: real-world documentation of functional class. J Med Econ. 2017;20(11):1178–86.
Masataka K, et al. Initial combination therapy of ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH) in the modified intention-to-treat population of the AMBITION study: post hoc analysis. Ann Rheumatic Dis. 2020;79(5):626–34.
Gaine S, et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J. 2017;50(2):111.
Pulido T, Adzerikho I, Channick R, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369:809–18.
Coghlan JG, Galie N, Barbera JA, et al. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis. 2017;76:1219–2122.
McLaughlin VV, Hoeper MM, Channick RN, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol. 2018;71(7):752–63. https://doi.org/10.1016/j.jacc.2017.12.010.
Acknowledgements
Funding
Funding for this study was provided Janssen Scientific Affairs, LLC, which participated in the design of the study, analysis, and interpretation of data, drafting, reviewing, and approving the publication. The journal’s Rapid Service Fee was funded by Janssen Scientific Affairs, LLC.
Medical Writing and Editorial Assistance
Medical writing assistance was provided by Risho Singh of STATINMED, LLC. Editorial assistance in the preparation of this article was provided by Christopher Moriarty of STATinMED, LLC. Funding for this assistance was provided by Janssen Scientific Affairs, LLC.
Author Contributions
Yuen Tsang, Risho Singh, Sumit Verma, Sumeet Panjabi contributed to the concept and design of the study, analysis and interpretation of the results, revisions of the manuscript, and maintained control over the final content.
Disclosures
Yuen Tsang and Sumeet Panjabi are employees of Janssen Scientific Affairs in Trenton, NJ, a subsidiary of Johnson and Johnson, the study sponsor. Risho Singh and Sumit Verma are the employees of STATinMED, LLC, a paid consultant to the study sponsors.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Since this study did not involve the collection, use, or transmittal of individually identifiable data, it was deemed exempt from Institutional Review Board review by Solutions IRB. Both the datasets and the security of the offices where analysis was completed (and where the datasets are kept) meet the requirements of the Health Insurance Portability and Accountability Act of 1996. Solutions IRB determined this study to be EXEMPT from the Office for Human Research Protections (OHRP)’s Regulations for the Protection of Human Subjects (45 CFR 46) under Exemption 4: Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. The HIPAA Authorization Waiver was granted in accordance with the specifications of 45 CFR 164.512(i). This project was conducted in full accordance with all applicable laws and regulations, and adhered to the project plan that was reviewed by Solutions Institutional Review Board.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to third-party license of Optum’s de-identified Clinformatics® Data Mart Database (2007-2021). As such, the authors cannot provide the raw data themselves. Other researchers could access these data by purchasing third-party licenses through these commercial data providers. Inclusion criteria specified in the Methods section would allow other researchers to identify the same cohort of patients we used for these analyses.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tsang, Y., Singh, R., Verma, S. et al. Hospitalization Among Pulmonary Arterial Hypertension Patients With and Without Connective Tissue Disease Comorbidities Prescribed Oral Selexipag. Rheumatol Ther 10, 741–756 (2023). https://doi.org/10.1007/s40744-023-00547-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00547-z