Abstract
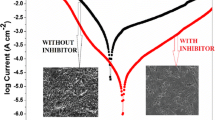
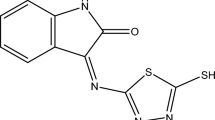
Due to the growing concern about corrosion problems caused by acidic attacks, the anticorrosive properties of synthetic corrosion inhibitor has been studied. The present work assessed the synthesis and inhibition performance of 3-((dicyclohexyl amino)methyl)–5–(4–((2-hydroxybenzylidene) amino phenyl)–1, 3, 4–oxadiazole–2(3H)–thione (DHOT) on the corrosion of mild steel in 1 M HCl solution through weight loss and electrochemical measurements. DHOT was characterized using FTIR and 1H NMR measurements. Surface analysis was carried out using SEM and AFM. Chemical theoretical studies of DHOT were performed using density functional theory (DFT) calculations. Electrochemical measurement results showed that corrosion inhibition increased as inhibitor concentration increased, achieving a significant inhibition efficiency of 98.86% at optimum conditions. Polarization curves indicated that molecules of DHOT acted as a mixed-type inhibitor, and their adsorption process obeyed the Langmuir isotherm. Examination of corroded steel surface by SEM and AFM confirmed the adsorption of DHOT on metal surface. Chemical quantum calculations were used to determine the electronic properties of the DHOT and to explain inhibitor/metal surface interactions.


















Similar content being viewed by others
Data Availability
All data are available in the research.
References
Berrissoul A, Ouarhach A, Benhiba F, Romane A, Zarrouk A, Guenbour A et al (2020) Evaluation of Lavandula mairei extract as green inhibitor for mild steel corrosion in 1 M HCl solution. Experimental and theoretical approach. J Mol Liq 313:113493. https://doi.org/10.1016/j.molliq.2020.113493
Rashid KH, Khadom AA (2020) 3-Methoxypropyl-amine as corrosion inhibitor for X80 steel in simulated saline water. J Mol Liq 319:114326. https://doi.org/10.1016/j.molliq.2020.114326
Khadom A, Rashid K (2018) Adsorption and kinetics behavior of kiwi juice as a friendly corrosion inhibitor of steel in acidic media. World J Eng 15(3):388–401. https://doi.org/10.1108/WJE-08-2017-0246
Fadhil AA, Khadom AA, Ahmed SK, Liu H, Fu C, Mahood HB (2020) Portulaca grandiflora as new green corrosion inhibitor for mild steel protection in hydrochloric acid: quantitative, electrochemical, surface and spectroscopic investigations. Surf Interfaces 20:100595. https://doi.org/10.1016/j.surfin.2020.100595
Khadom AA (2015) Kinetics and synergistic effect of iodide ion and naphthylamine for the inhibition of corrosion reaction of mild steel in hydrochloric acid. React Kinet Mech Catal 115(2):463–481. https://doi.org/10.1007/s11144-015-0873-9
Khadom AA, Yaro AS (2011) Modeling of corrosion inhibition of copper-nickel alloy in hydrochloric acid by benzotriazole. Russ J Phys Chem A 85(11):2005–2012. https://doi.org/10.1134/S0036024411110148
Shahini MH, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B (2021) Superior inhibition action of the Mish Gush (MG) leaves extract toward mild steel corrosion in HCl solution: theoretical and electrochemical studies. J Mol Liq 332:115876. https://doi.org/10.1016/j.molliq.2021.115876
Swathi NP, Samshuddin S, Aljohani TA, Rasheeda K, Alva VD, Alomari FY, Alamri AH (2022) A new 1, 2, 4-triazole derivative as an excellent corrosion inhibitor: electrochemical experiments with theoretical validation. Mater Chem Phys 291:126677. https://doi.org/10.1016/j.matchemphys.2022.126677
Fouda AEAS, Etaiw SEH, Ismail MA, Abd El-Aziz DM, Eladl MM (2022) Novel naphthybithiophene derivatives as corrosion inhibitors for carbon steel in 1 M HCl: electrochemical, surface characterization and computational approaches. J Mol Liq 367:120394. https://doi.org/10.1016/j.molliq.2022.120394
Al-Uqaily RA, Al-Bayaty SA, Khadom AA, Kadhim MM (2022) Inhibitive performance of 4-Methoxyphenethylamine on low-carbon steel in 1 M hydrochloric acid: kinetics, theoretical, and mathematical views. J Mol Liq 350:118523. https://doi.org/10.1016/j.molliq.2022.118523
Carlos MF, Barboza GK, Echevarria A (2022) Anticorrosive effect of halogenated aniline enaminoesters on carbon steel in HCl. Int J Corros. https://doi.org/10.1155/2022/7218063
Khadom AA, Yaro AS (2011) Protection of low carbon steel in phosphoric acid by potassium iodide. Prot Met Phys Chem Surf 47:662–669. https://doi.org/10.1134/S2070205111050078
Elabbasy HM, Gadow HS (2021) Study the effect of expired tenoxicam on the inhibition of carbon steel corrosion in a solution of hydrochloric acid. J Mol Liq 321:114918. https://doi.org/10.1016/j.molliq.2020.114918
Selatnia I, Sid A, Benahmed M, Ozturk T, Gherraf N (2018) Synthesis and characterization of a bis-pyrazoline derivative as corrosion inhibitor for A283 carbon steel in 1M HCl: electrochemical, surface, DFT and MD simulation studies. Prot Met Phys Chem Surf 54(6):1182–1193. https://doi.org/10.1134/S2070205118060229
Sehmi A, Ouici HB, Guendouzi A, Ferhat M, Benali O, Boudjellal F (2020) Corrosion inhibition of mild steel by newly synthesized pyrazole carboxamide derivatives in HCl acid medium: experimental and theoretical studies. J Electrochem Soc 167(15):155508. https://doi.org/10.1149/1945-7111/abab25
Mrani SA, El Arrouji S, Karrouchi K, El Hajjaji F, Alaoui KI, Rais Z, Taleb M (2018) Inhibitory performance of some pyrazole derivatives against corrosion of mild steel in 1.0 M HCl: electrochemical, MEB and theoretical studies. Int J Corros Scale Inhib 7(4):542–569. https://doi.org/10.17675/2305-6894-2018-7-4-5
Laadam G, Benhiba F, El Faydy M, Titi A, Al-Gorair AS, Alshareef M et al (2022) Anti-corrosion performance of novel pyrazole derivative for carbon steel corrosion in 1 M HCl: Computational and experimental studies. Inorg Chem Commun 145:109963. https://doi.org/10.1016/j.inoche.2022.109963
Grudic V, Boskovic I, Radonjic D, Jacimovic Z, Knezevic B (2019) The electrochemical behavior of Al alloys in NaCl solution in the presence of pyrazole derivative. Iran J Chem Chem Eng (IJCCE) 38(2):127–138. https://doi.org/10.30492/IJCCE.2019.30659
Singh A, Ansari KR, Chauhan DS, Quraishi MA, Lgaz H, Chung IM (2020) Comprehensive investigation of steel corrosion inhibition at macro/micro level by ecofriendly green corrosion inhibitor in 15% HCl medium. J Colloid Interface Sci 560:225–236. https://doi.org/10.1016/j.jcis.2019.10.040
Ahmed SK, Ali WB, Khadom AA (2019) Synthesis and characterization of new triazole derivatives as corrosion inhibitors of carbon steel in acidic medium. J Bio Tribo Corros 5(1):15. https://doi.org/10.1007/s40735-018-0209-1
Abdulridha AA, Allah MAAH, Makki SQ, Sert Y, Salman HE, Balakit AA (2020) Corrosion inhibition of carbon steel in 1 M H2SO4 using new Azo Schiff compound: electrochemical, gravimetric, adsorption, surface and DFT studies. J Mol Liq 315:113690. https://doi.org/10.1016/j.molliq.2020.113690
El Aatiaoui A, Koudad M, Chelfi T, Erkan S, Azzouzi M, Aouniti A et al (2021) Experimental and theoretical study of new Schiff bases based on imidazo (1, 2-a) pyridine as corrosion inhibitor of mild steel in 1M HCl. J Mol Struct 1226:129372. https://doi.org/10.1016/j.molstruc.2020.129372
Sayed AR, El-Lateef HMA (2020) Thiocarbohydrazones based on adamantane and ferrocene as efficient corrosion Inhibitors for hydrochloric acid pickling of C-steel. Coatings 10(11):1068. https://doi.org/10.3390/coatings10111068
Shenoy KV, Venugopal PP, Kumari PR, Chakraborty D (2021) Effective inhibition of mild steel corrosion by 6-bromo-(2, 4-dimethoxyphenyl) methylidene] imidazo [1, 2-a] pyridine-2-carbohydrazide in 0.5 M HCl: Insights from experimental and computational study. J Mol Struct 1232:130074. https://doi.org/10.1016/j.molstruc.2021.130074
Al-Azawi KF, Mohammed IM, Al-Baghdadi SB, Salman TA, Issa HA, Al-Amiery AA et al (2018) Experimental and quantum chemical simulations on the corrosion inhibition of mild steel by 3-((5-(3, 5-dinitrophenyl)-1, 3, 4-thiadiazol-2-yl) imino) indolin-2-one. Results Phys 9:278–283. https://doi.org/10.1016/j.rinp.2018.02.055
Farj AS, AL-Azawi KF (2022) Synthesis, characterization and antimicrobial activity of novel Mannich bases. AIP Conf Proc 2437:020090. https://doi.org/10.1063/5.0092314
Ozyazici T, Gurdal EE, Orak D, Sipahi H, Ercetin T, Gulcan HO, Koksal M (2020) Synthesis, anti-inflammatory activity, and molecular docking studies of some novel Mannich bases of the 1,3,4-oxadiazole-2(3H)-thione scaffold. Arch Pharm 353(7):1–16. https://doi.org/10.1002/ardp.202000061
Hussain Z, Yousif E, Ahmed A, Altaie A (2014) Synthesis and characterization of Schiff’s bases of sulfamethoxazole. Org Med Chem Lett 4(1):1. https://doi.org/10.1186/2191-2858-4-1
Fernandes CM, Alvarez LX, dos Santos NE, Barrios ACM, Ponzio EA (2019) Green synthesis of 1-benzyl-4-phenyl-1H-1, 2, 3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros Sci 149:185–194. https://doi.org/10.1016/j.corsci.2019.01.019
Rashid KH, Khadom AA, Abbas SH (2022) Optimization, kinetics, and electrochemical investigations for green corrosion inhibition of low-carbon steel in 1 M HCl by a blend of onion-garlic leaves wastes. Bioresour Technol Reports 19:101194. https://doi.org/10.1016/j.biteb.2022.101194
Rbaa M, Benhiba F, Dohare P, Lakhrissi L, Touir R, Lakhrissi B et al (2020) Synthesis of new epoxy glucose derivatives as a non-toxic corrosion inhibitors for carbon steel in molar HCl: experimental, DFT and MD simulation. Chem Data Collect 27:100394. https://doi.org/10.1016/j.cdc.2020.100394
Alkarim TA, Al-Azawi KF, Anaee RA (2021) Anticorrosive properties of Spiramycin for aluminum in acidic medium. Int J Corros Scale Inhib 10(3):1168–1188. https://doi.org/10.17675/2305-6894-2021-10-3-20
El Arrouji S, Karrouchi K, Berisha A, Alaoui KI, Warad I, Rais Z et al (2020) New pyrazole derivatives as effective corrosion inhibitors on steel-electrolyte interface in 1 M HCl: electrochemical, surface morphological (SEM) and computational analysis. Colloids Surf A Physicochem Eng Aspects 604:125325. https://doi.org/10.1016/j.colsurfa.2020.125325
Jasim AS, Rashid KH, AL-Azawi KF, Khadom AA (2022) Synthesis of a novel pyrazole heterocyclic derivative as corrosion inhibitor for low-carbon steel in 1M HCl: characterization, gravimetrical, electrochemical, mathematical, and quantum chemical investigations. Results Eng 15:100573. https://doi.org/10.1016/J.RINENG.2022.100573
Saranya J, Sowmiya M, Sounthari P, Parameswari K, Chitra S, Senthilkumar K (2016) N-heterocycles as corrosion inhibitors for mild steel in acid medium. J Mol Liq 216:42–52. https://doi.org/10.1016/j.molliq.2015.12.096
Rashid KH, AL-Azawi KF, Khadom AA, Jasim AS, Kadhim MM (2023) New pyrazole derivative as effective corrosion inhibitor for carbon steel in 1 M HCl: experimental and theoretical analysis. J Mol Struct. https://doi.org/10.1016/j.molstruc.2023.135661
Akinbulumo OA, Odejobi OJ, Odekanle EL (2020) Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results Mater 5:100074. https://doi.org/10.1016/j.rinma.2020.100074
Jadaa RJ, Abd AN, Khadom AA (2021) Polyacrylamide as a corrosion inhibitor for mild steel in 2 M phosphoric acid: experimental and theoretical studies. Chem Pap 75:5375–5386. https://doi.org/10.1007/s11696-021-01725-5
Sowmyashree AS, Somya A, Rao S, Kumar CP, Al-Romaizan AN, Hussein MA et al (2023) Potential sustainable electrochemical corrosion inhibition study of Citrus limetta on mild steel surface in aggressive acidic media. J Market Res 24:984–994. https://doi.org/10.1016/j.jmrt.2023.02.039
Fernandes CM, Costa AR, Leite MC, Martins V, Lee HS, da CS Boechat F et al (2023) A detailed experimental performance of 4-quinolone derivatives as corrosion inhibitors for mild steel in acid media combined with first-principles DFT simulations of bond breaking upon adsorption. J Mol Liq. https://doi.org/10.1016/j.molliq.2023.121299
Hajjaji FE, Ech-chihbi E, Salim R, Titi A, Messali M, El Ibrahimi B et al (2023) A detailed electronic-scale DFT modeling/MD simulation, electrochemical and surface morphological explorations of imidazolium-based ionic liquids as sustainable and non-toxic corrosion inhibitors for mild steel in 1 M HCl. Mater Sci Eng, B 289:116232. https://doi.org/10.1016/j.mseb.2022.116232
Jasim AS, Khadom AA, Rashid KH, AL-Azawi KF (2022) (3, 5-dimethyl-1H-pyrazol-1-y1)(4-((3, 4-dimethoxybenzylidene) amino) phenyl) methanone as a novel corrosion inhibitor for low-carbon steel in hydrochloric acid: Synthesis, diagnosis, and application. Results Chem 4:100569. https://doi.org/10.1016/j.rechem.2022.100569
Swetha GA, Sachin HP, Guruprasad AM, Prasanna BM, Sudheer Kumar KH (2018) Use of seroquel as an effective corrosion inhibitor for low carbon steel in 1 M HCl. J Bio Tribo Corros 4:1–11. https://doi.org/10.1007/s40735-018-0173-9
Sundaram RG, Vengatesh G, Sundaravadivelu M (2021) Surface morphological and quantum chemical studies of some expired drug molecules as potential corrosion inhibitors for mild steel in chloride medium. Surf Interfaces 22:100841. https://doi.org/10.1016/j.surfin.2020.100841
Alaoui K, Touir R, Galai M, Serrar H, Ouakki M, Kaya S et al (2018) Electrochemical and computational studies of some triazepine carboxylate compounds as acid corrosion inhibitors for mild steel. J Bio Tribo Corros 4:1–18. https://doi.org/10.1007/s40735-018-0154-z
El-Hajjaji F, Messali M, Aljuhani A, Aouad MR, Hammouti B, Belghiti ME et al (2018) Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: electrochemical and molecular dynamics simulation studies. J Mol Liq 249:997–1008. https://doi.org/10.1016/j.molliq.2017.11.111
Asaad MA, Sarbini NN, Sulaiman A, Ismail M, Huseien GF, Majid ZA, Raja PB (2018) Improved corrosion resistance of mild steel against acid activation: impact of novel Elaeis guineensis and silver nanoparticles. J Ind Eng Chem 63:139–148. https://doi.org/10.1016/j.jiec.2018.02.010
Edison TNJI, Atchudan R, Pugazhendhi A, Lee YR, Sethuraman MG (2018) Corrosion inhibition performance of spermidine on mild steel in acid media. J Mol Liq 264:483–489. https://doi.org/10.1016/j.molliq.2018.05.087
Haldhar R, Prasad D, Saxena A, Kumar R (2018) Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. Sustain Chem Pharm 9:95–105. https://doi.org/10.1016/j.scp.2018.07.002
Chauhan DS, Ansari KR, Sorour AA, Quraishi MA, Lgaz H, Salghi R (2018) Thiosemicarbazide and thiocarbohydrazide functionalized chitosan as ecofriendly corrosion inhibitors for carbon steel in hydrochloric acid solution. Int J Biol Macromol 107:1747–1757. https://doi.org/10.1016/j.ijbiomac.2017.10.050
Hamani H, Douadi T, Daoud D, Al-Noaimi M, Rikkouh RA, Chafaa S (2017) 1-(4-Nitrophenylo-imino)-1-(phenylhydrazono)-propan-2-one as corrosion inhibitor for mild steel in 1 M HCl solution: weight loss, electrochemical, thermodynamic and quantum chemical studies. J Electroanal Chem 801:425–438. https://doi.org/10.1016/j.jelechem.2017.08.031
Abd El-Lateef HM, Tantawy AH, Abdelhamid AA (2017) Novel quaternary ammonium-based cationic surfactants: synthesis, surface activity and evaluation as corrosion inhibitors for C1018 carbon steel in acidic chloride solution. J Surfactants Deterg 20:735–753. https://doi.org/10.1007/s11743-017-1947-7
Salhi A, Tighadouini S, El-Massaoudi M, Elbelghiti M, Bouyanzer A, Radi S et al (2017) Keto-enol heterocycles as new compounds of corrosion inhibitors for carbon steel in 1 M HCl: weight loss, electrochemical and quantum chemical investigation. J Mol Liq 248:340–349. https://doi.org/10.1016/j.molliq.2017.10.040
Yan Y, Lin X, Zhang L, Zhou H, Wu L, Cai L (2017) Electrochemical and quantum-chemical study on newly synthesized triazoles as corrosion inhibitors of mild steel in 1 M HCl. Res Chem Intermed 43:3145–3162. https://doi.org/10.1007/s11164-016-2816-0
Nathiya RS, Perumal S, Moorthy M, Murugesan V, Rangappan R, Raj V (2020) Synthesis, characterization and inhibition performance of schiff bases for aluminium corrosion in 1 MH 2 SO 4 solution. J Bio Tribo Corros 6:1–15. https://doi.org/10.1007/s40735-019-0291-z
Haque J, Srivastava V, Verma C, Quraishi MA (2017) Experimental and quantum chemical analysis of 2-amino-3-((4-((S)-2-amino-2-carboxyethyl)-1H-imidazol-2-yl) thio) propionic acid as new and green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution. J Mol Liq 225:848–855. https://doi.org/10.1016/j.molliq.2016.11.011
Anusuya N, Saranya J, Sounthari P, Zarrouk A, Chitra S (2017) Corrosion inhibition and adsorption behaviour of some bis-pyrimidine derivatives on mild steel in acidic medium. J Mol Liq 225:406–417. https://doi.org/10.1016/j.molliq.2016.11.015
Salarvand Z, Amirnasr M, Talebian M, Raeissi K, Meghdadi S (2017) Enhanced corrosion resistance of mild steel in 1 M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives: experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros Sci 114:133–145. https://doi.org/10.1016/j.corsci.2016.11.002
Hameed WF, Rashid KH, Khadom AA (2022) Investigation of tetraazaadamantane as corrosion inhibitor for mild steel in oilfield produced water under sweet corrosive environment. J Bio Tribo Corros 8(1):27. https://doi.org/10.1007/s40735-021-00626-0
Laggoun R, Ferhat M, Saidat B, Benghia A, Chaabani A (2020) Effect of p-toluenesulfonyl hydrazide on copper corrosion in hydrochloric acid solution. Corros Sci 165:108363. https://doi.org/10.1016/j.corsci.2019.108363
Xu B, Yang W, Liu Y, Yin X, Gong W, Chen Y (2014) Experimental and theoretical evaluation of two pyridinecarboxaldehyde thiosemicarbazone compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 78:260–268. https://doi.org/10.1016/j.corsci.2013.10.007
Wang X, Yang H, Wang F (2011) An investigation of benzimidazole derivative as corrosion inhibitor for mild steel in different concentration HCl solutions. Corros Sci 53(1):113–121. https://doi.org/10.1016/j.corsci.2010.09.029
Goyal M, Kumar S, Bahadur I, Verma C, Ebenso EE (2018) Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: a review. J Mol Liq 256:565–573. https://doi.org/10.1016/j.molliq.2018.02.045
Singh P, Quraishi MA, Gupta SL, Dandia A (2016) Investigation of the corrosion inhibition effect of 3-methyl-6-oxo-4-(thiophen-2-yl)-4,5,6,7-tetrahydro-2H pyrazolo [3,4-b]pyridine-5-carbonitrile (TPP) on mild steel in hydrochloric acid. J Taibah Univ Sci 10:139–147. https://doi.org/10.1016/j.jtusci.2015.07.005
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
ZIJ: data collection. KHR: conceptualization and methodology, and software. KFAL-A: synthesis of corrosion inhibitor and diagnosis, AAK: supervision, analysis, software, discussion, writing, reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jasim, Z.I., Rashid, K.H., AL-Azawi, K.F. et al. Synthesis of Schiff-Based Derivative as a Novel Corrosion Inhibitor for Mild Steel in 1 M HCl Solution: Optimization, Experimental, and Theoretical Investigations. J Bio Tribo Corros 9, 54 (2023). https://doi.org/10.1007/s40735-023-00774-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-023-00774-5