Abstract
Purpose of Review
The increasing occurrence of emerging chemicals of concern in the environment has caused high public attention. Assessing their hematologic toxicities is of high priority, as the blood circulation system is usually essential in transporting these exogenous substances to diverse target tissues in vivo. The plasma kallikrein-kinin system (KKS) is one of the most abundant protease enzyme systems and regulates a series of crucial hematologic functions. As a vulnerable target, the KKS may sensitively respond to circulatory pollutants, and combing the current studies on the interaction of the environmental contaminants with the KKS would help understand the toxicological or pathological significance of this system.
Recent Findings
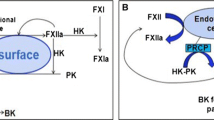
The current studies have revealed that some environmental contaminants, such as small molecular organic chemicals, engineered nanoparticles (NPs), and atmospheric fine particulate matter (PM), can directly interact with the KKS, causing the autoactivation of the Hageman factor XII (FXII), the subsequent cascade cleavage of the plasma prekallikrein (PPK), and high molecular kininogen (HK). The consequent downstream hematological effects and other related toxicities can be concomitantly induced via the crosstalk with the KKS. In addition, multiple approaches, based on in vitro, ex vivo, and in vivo experimental models, have been developed to characterize the binding of exogenous substances with FXII, conformational changes of the protein, the cascade activation of the KKS, and downstream toxicological or pathological responses.
Summary
As a vulnerable target, the plasma KKS sensitively responds to the exposure of environmental pollutants and is promising for biomonitoring hematotoxicity in future studies.

Similar content being viewed by others
Data Availability
The data reported in this review paper are based on raw data available in the published literature.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Pathak M, Wong SS, Dreveny I, Emsley J. Structure of plasma and tissue kallikreins. Thromb Haemost. 2013;110(3):423–33. https://doi.org/10.1160/th12-11-0840.
Naudin C, Burillo E, Blankenberg S, Butler L, Renné T. Factor XII contact activation. Semin Thromb Hemost. 2017;43(8):814–26. https://doi.org/10.1055/s-0036-1598003.
Burch RM. Bradykinin Receptors. In: Lennarz WJ, Lane MD, editors. Encyclopedia of biological chemistry. 2nd ed. Waltham: Academic Press; 2013.
Clements JA, Willemsen NM, Myers SA, Dong Y. The tissue kallikrein family of serine proteases: functional roles in human disease and potential as clinical biomarkers. Crit Rev Clin Lab Sci. 2004;41(3):265–312. https://doi.org/10.1080/10408360490471931.
Yousef GM, Obiezu CV, Luo LY, Magklara A, Borgoño CA, Kishi T, et al. Human tissue kallikreins: from gene structure to function and clinical applications. Adv Clin Chem. 2005;39:11–79. https://doi.org/10.1016/s0065-2423(04)39002-5.
Skidgel RA, Erdös EG, Deddish PA. Kininases. In: Martini L, editor. Encyclopedia of endocrine diseases. New York: Elsevier; 2004.
Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90(10):3819–43.
Costa-Neto CM, Dillenburg-Pilla P, Heinrich TA, Parreiras-e-Silva LT, Pereira MGAG, Reis RI, et al. Participation of kallikrein–kinin system in different pathologies. Int Immunopharmacol. 2008;8(2):135–42. https://doi.org/10.1016/j.intimp.2007.08.003.
Nigretto JM, Corretge E, Jozefowicz M. Contributions of negatively charged chemical groups to the surface-dependent activation of human plasma by soluble dextran derivatives. Biomaterials. 1989;10(7):449–54. https://doi.org/10.1016/0142-9612(89)90085-9.
Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14(1):28–39. https://doi.org/10.1111/jth.13194.
Konrath S, Mailer RK, Renné T. Mechanism, functions, and diagnostic relevance of FXII activation by foreign surfaces. Hamostaseologie. 2021;41(6):489–501. https://doi.org/10.1055/a-1528-0499.
Davoine C, Bouckaert C, Fillet M, Pochet L. Factor XII/XIIa inhibitors: their discovery, development, and potential indications. Eur J Med Chem. 2020;208: 112753. https://doi.org/10.1016/j.ejmech.2020.112753.
Zhuo R, Siedlecki CA, Vogler EA. Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces. Biomaterials. 2006;27(24):4325–32. https://doi.org/10.1016/j.biomaterials.2006.04.001.
Engel R, Brain CM, Paget J, Lionikiene AS, Mutch NJ. Single-chain factor XII exhibits activity when complexed to polyphosphate. J Thromb Haemost. 2014;12(9):1513–22. https://doi.org/10.1111/jth.12663.
Yan Y, Xu LC, Vogler EA, Siedlecki CA. 1 - Contact activation by the intrinsic pathway of blood plasma coagulation. In: Siedlecki CA, editor. Hemocompatibility of biomaterials for clinical applications. Woodhead Publishing; 2018.
Ishihara K, Kamata M, Hayashi I, Yamashina S, Majima M. Roles of bradykinin in vascular permeability and angiogenesis in solid tumor. Int Immunopharmacol. 2002;2(4):499–509. https://doi.org/10.1016/S1567-5769(01)00193-X.
Maas C, Renné T. Coagulation factor XII in thrombosis and inflammation. Blood. 2018;131(17):1903–9. https://doi.org/10.1182/blood-2017-04-569111.
Björkqvist J, Jämsä A, Renné T. Plasma kallikrein: the bradykinin-producing enzyme. Thromb Haemost. 2013;110(3):399–407. https://doi.org/10.1160/th13-03-0258.
Morais RL, Silva ED, Sales VM, Filippelli-Silva R, Mori MA, Bader M, et al. Kinin B1 and B2 receptor deficiency protects against obesity induced by a high-fat diet and improves glucose tolerance in mice. Diabet Metab Synd Ob. 2015;8:399–407. https://doi.org/10.2147/DMSO.S87635.
Kalinin DV. Factor XII(a) inhibitors: a review of the patent literature. Expert Opin Ther Pat. 2021;31(12):1155–76. https://doi.org/10.1080/13543776.2021.1945580.
Ivanov I, Verhamme IM, Sun MF, Mohammed B, Cheng Q, Matafonov A, et al. Protease activity in single-chain prekallikrein. Blood. 2020;135(8):558–67. https://doi.org/10.1182/blood.2019002224.
Joseph K, Kaplan AP. Formation of bradykinin: a major contributor to the innate inflammatory response. In: Alt FW, editor. Advances in Immunology. Academic Press; 2005.
Campbell DJ. Chapter 188 - Bradykinin Peptides. In: Kastin AJ, editor. Handbook of biologically active peptides. 2nd ed. Boston: Academic Press; 2013.
Girolami A, Scarparo P, Candeo N, Lombardi AM. Congenital prekallikrein deficiency. Expert Rev Hematol. 2010;3(6):685–95. https://doi.org/10.1586/ehm.10.69.
Kaplan AP, Ghebrehiwet B. The plasma bradykinin-forming pathways and its interrelationships with complement. Mol Immunol. 2010;47(13):2161–9. https://doi.org/10.1016/j.molimm.2010.05.010.
Renné T, Gailani D, Meijers JCM, Müller-Esterl W. Characterization of the H-kininogen-binding site on factor XI: a comparison of factor XI and plasma prekallikrein. J Biol Chem. 2002;277(7):4892–9. https://doi.org/10.1074/jbc.M105221200.
Kearney KJ, Butler J, Posada OM, Wilson C, Heal S, Ali M, et al. Kallikrein directly interacts with and activates factor IX, resulting in thrombin generation and fibrin formation independent of factor XI. PNAS. 2021;118(3): e2014810118. https://doi.org/10.1073/pnas.2014810118.
Cassaro CM, Sampaio MU, Maeda NY, Chamone DF, Sampaio CA. Human plasma kallikrein: effect on the induced platelet aggregation. Thromb Res. 1987;48(1):81–7. https://doi.org/10.1016/0049-3848(87)90348-3.
Wong MKS. Subchapter 30B - Kallikrein. In: Takei Y, Ando H, Tsutsui K, editors. Handbook of hormones. San Diego: Academic Press; 2016.
Wong MKS. Subchapter 43A - Kininogen. In: Ando H, Ukena K, Nagata S, editors. Handbook of hormones. 2nd ed. San Diego: Academic Press; 2021.
Puri RN, Zhou F, Hu C, Colman RF, Colman RW. High molecular weight kininogen inhibits thrombin-induced platelet aggregation and cleavage of aggregin by inhibiting binding of thrombin to platelets. Blood. 1991;77(3):500–7.
Bennett V. Bradykinin. In: Enna SJ, Bylund DB, editors. xPharm: the comprehensive pharmacology reference. New York: Elsevier; 2007.
Cyr M, Lepage Y, Blais C Jr, Gervais N, Cugno M, Rouleau JL, et al. Bradykinin and des-Arg(9)-bradykinin metabolic pathways and kinetics of activation of human plasma. Am J Physiol Heart Circ Physiol. 2001;281(1):H275–83. https://doi.org/10.1152/ajpheart.2001.281.1.H275.
Wong MKS. Subchapter 42D - angiotensin converting enzyme. In: Ando H, Ukena K, Nagata S, editors. Handbook of hormones. 2nd ed. San Diego: Academic Press; 2021.
Sharma J. Activation of the bradykinin system by angiotensin-converting enzyme inhibitors. Eur J Inflamm. 2010;8:55–61. https://doi.org/10.1177/1721727X1000800201.
Ehrenfeld P, Manso L, Pavicic MF, Matus CE, Borquez C, Lizama A, et al. Bioregulation of kallikrein-related peptidases 6, 10 and 11 by the kinin B1 receptor in breast cancer cells. Anticancer Res. 2014;34(12):6925–38.
Yin Y, Ye C, Zhou F, Wang J, Yang D, Yin W, et al. Molecular basis for kinin selectivity and activation of the human bradykinin receptors. Nat Str Uct Mol Biol. 2021;28(9):755–61. https://doi.org/10.1038/s41594-021-00645-y.
Shen J, Zhang H. Function and structure of bradykinin receptor 2 for drug discovery. Acta Pharmacol Sin. 2022. https://doi.org/10.1038/s41401-022-00982-8.
Maestri R, Milia AF, Salis MB, Graiani G, Lagrasta C, Monica M, et al. Cardiac hypertrophy and microvascular deficit in kinin B2 receptor knockout mice. Hypertension. 2003;41(5):1151–5. https://doi.org/10.1161/01.Hyp.0000064180.55222.Df.
Pfeffer MA. Heart failure and hypertension: importance of prevention. Med Clin North Am. 2017;101(1):19–28. https://doi.org/10.1016/j.mcna.2016.08.012.
McCarty MF. ACE inhibition may decrease diabetes risk by boosting the impact of bradykinin on adipocytes. Med Hypotheses. 2003;60(6):779–83. https://doi.org/10.1016/s0306-9877(02)00234-7.
Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343(1):227–35. https://doi.org/10.1007/s00441-010-1034-0.
Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–738. https://doi.org/10.1152/physrev.00038.2017.
Bekassy Z, Lopatko Fagerström I, Bader M, Karpman D. Crosstalk between the renin–angiotensin, complement and kallikrein–kinin systems in inflammation. Nat Rev Immunol. 2022;22(7):411–28. https://doi.org/10.1038/s41577-021-00634-8.
Shariat-Madar Z, Mahdi F, Schmaier AH. Assembly and activation of the plasma kallikrein/kinin system: a new interpretation. Int Immunopharmacol. 2002;2(13):1841–9. https://doi.org/10.1016/S1567-5769(02)00178-9.
Vogler EA, Siedlecki CA. Contact activation of blood-plasma coagulation. Biomaterials. 2009;30(10):1857–69. https://doi.org/10.1016/j.biomaterials.2008.12.041.
Kashuba E, Bailey J, Allsup D, Cawkwell L. The kinin-kallikrein system: physiological roles, pathophysiology and its relationship to cancer biomarkers. Biomarkers. 2013;18(4):279–96. https://doi.org/10.3109/1354750X.2013.787544.
Chandler WL. Chapter 145 - Fibrinolytic Testing. In: Shaz BH, Hillyer CD, Reyes Gil M, editors. Transfusion medicine and hemostasis. 3rd ed. Elsevier; 2019.
Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29(1):17–24. https://doi.org/10.1016/j.blre.2014.09.003.
Binnema DJ, Dooijewaard G, Turion PN. An analysis of the activators of single-chain urokinase-type plasminogen activator (scu-PA) in the dextran sulphate euglobulin fraction of normal plasma and of plasmas deficient in factor XII and prekallikrein. Thromb Haemost. 1991;65(2):144–8.
Pahlavani M, Kalupahana NS, Ramalingam L, Moustaid-Moussa N. Regulation and functions of the renin-angiotensin system in white and brown adipose tissue. Compr Physiol. 2017;7(4):1137–50. https://doi.org/10.1002/cphy.c160031.
Yim HE, Yoo KH. Renin-angiotensin system - considerations for hypertension and kidney. Electrolyte Blood Press; 2008.
Yang Y, Liu G, He Q, Shen J, Xu L, Zhu P, et al. A promising candidate: heparin-binding protein steps onto the stage of sepsis prediction. J Immunol Res. 2019;2019:7515346. https://doi.org/10.1155/2019/7515346.
Bentzer P, Fisher J, Kong HJ, Mörgelin M, Boyd JH, Walley KR, et al. Heparin-binding protein is important for vascular leak in sepsis. Intensive Care Med Exp. 2016;4(1):33. https://doi.org/10.1186/s40635-016-0104-3.
Kenne E, Soehnlein O, Herwald H, Lindbom L. Neutrophil-derived heparin binding protein (HBP) is an endogenous activator of the kallikrein-kinin system. FASEB J. 2009;23(S1):762.3. https://doi.org/10.1096/fasebj.23.1_supplement.762.3.
Alvarenga PH, Xu X, Oliveira F, Chagas AC, Nascimento CR, Francischetti IM, et al. Novel family of insect salivary inhibitors blocks contact pathway activation by binding to polyphosphate, heparin, and dextran sulfate. Arterioscler Thromb Vasc Biol. 2013;33(12):2759–70. https://doi.org/10.1161/atvbaha.113.302482.
Bender L, Weidmann H, Rose-John S, Renné T, Long AT. Factor XII-driven inflammatory reactions with implications for anaphylaxis. Front Immunol. 2017;8:1115. https://doi.org/10.3389/fimmu.2017.01115.
Gomez-Garcia MJ, Doiron AL, Steele RRM, Labouta HI, Vafadar B, Shepherd RD, et al. Nanoparticle localization in blood vessels: dependence on fluid shear stress, flow disturbances, and flow-induced changes in endothelial physiology. Nanoscale. 2018;10(32):15249–61. https://doi.org/10.1039/c8nr03440k.
Duffek A, Conrad A, Kolossa-Gehring M, Lange R, Rucic E, Schulte C, et al. Per- and polyfluoroalkyl substances in blood plasma – results of the German Environmental Survey for children and adolescents 2014–2017 (GerES V). Int J Hyg Environ Health. 2020;228: 113549. https://doi.org/10.1016/j.ijheh.2020.113549.
Vlaanderen JJ, Janssen NA, Hoek G, Keski-Rahkonen P, Barupal DK, Cassee FR, et al. The impact of ambient air pollution on the human blood metabolome. Environ Res. 2017;156:341–8. https://doi.org/10.1016/j.envres.2017.03.042.
Wang H, Zhang J, Ye L, Li S, Wang F, Zha W, et al. Plasma kallikrein-kinin system mediates immune-mediated renal injury in trichloroethylene-sensitized mice. J Immunotoxicol. 2016;13(4):567–79. https://doi.org/10.3109/1547691x.2016.1142019.
Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, et al. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ Sci Process Impacts. 2020;22(12):2345–73. https://doi.org/10.1039/d0em00291g.
Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. Per- and polyfluoroalkyl substance toxicity and human healthreview: current state of knowledge and strategies for informing future research. Environ Toxicol Chem. 2021;40(3):606–30. https://doi.org/10.1002/etc.4890.
•• Liu QS, Sun Y, Qu G, Long Y, Zhao X, Zhang A, et al. Structure-dependent hematological effects of per- and polyfluoroalkyl substances on activation of plasma kallikrein–kinin system cascade. Environ Sci Technol. 2017;51(17):10173–83. https://doi.org/10.1021/acs.est.7b02055. This article shows for the first time that small molecule organic compounds of PFASs can activate plasma KKS.
Liu QS, Hao F, Sun Z, Long Y, Zhou Q, Jiang G. Perfluorohexadecanoic acid increases paracellular permeability in endothelial cells through the activation of plasma kallikrein-kinin system. Chemosphere. 2018;190:191–200. https://doi.org/10.1016/j.chemosphere.2017.10.002.
Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H, Spiccia L. Nanomaterials: applications in cancer imaging and therapy. Adv Mater. 2011;23(12):H18-40. https://doi.org/10.1002/adma.201100140.
Joudeh N, Linke D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnology. 2022;20(1):262. https://doi.org/10.1186/s12951-022-01477-8.
Yin L, Zhong Z. 1.3.8B - Nanoparticles. In: Science Biomaterials, editor. Wagner WR, Sakiyama-Elbert SE, Zhang G, Yaszemski MJ. 4th ed. Academic Press; 2020.
Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Impact of nanoparticles on brain health: an up to date overview. J Clin Med. 2018;7(12). https://doi.org/10.3390/jcm7120490.
Ngo W, Ahmed S, Blackadar C, Bussin B, Ji Q, Mladjenovic SM, et al. Why nanoparticles prefer liver macrophage cell uptake in vivo. Adv Drug Deliv Rev. 2022;185: 114238. https://doi.org/10.1016/j.addr.2022.114238.
LoPresti ST, Arral ML, Chaudhary N, Whitehead KA. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J Control Release. 2022;345:819–31. https://doi.org/10.1016/j.jconrel.2022.03.046.
Gatti AM, Montanari S, Ferrero S, Lavezzi AM. Silver nanoparticles in the fetal brain: new perspectives in understanding the pathogenesis of unexplained stillbirths. Nanomedicine (Lond). 2021;16(4):265–74. https://doi.org/10.2217/nnm-2020-0391.
Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–81. https://doi.org/10.1038/nnano.2013.181.
Lundqvist M, Augustsson C, Lilja M, Lundkvist K, Dahlbäck B, Linse S, et al. The nanoparticle protein corona formed in human blood or human blood fractions. PLoS ONE. 2017;12(4): e0175871. https://doi.org/10.1371/journal.pone.0175871.
Albanese A, Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Secreted biomolecules alter the biological identity and cellular interactions of nanoparticles. ACS Nano. 2014;8(6):5515–26. https://doi.org/10.1021/nn4061012.
Escamilla-Rivera V, Solorio-Rodríguez A, Uribe-Ramírez M, Lozano O, Lucas S, Chagolla-López A, et al. Plasma protein adsorption on Fe3O4-PEG nanoparticles activates the complement system and induces an inflammatory response. Int J Nanomedicine. 2019;14:2055–67. https://doi.org/10.2147/ijn.S192214.
Ekdahl KN, Davoodpour P, Ekstrand-Hammarström B, Fromell K, Hamad OA, Hong J, et al. Contact (kallikrein/kinin) system activation in whole human blood induced by low concentrations of α-Fe2O3 nanoparticles. Nanomedicine. 2018;14(3):735–44. https://doi.org/10.1016/j.nano.2017.12.008.
Ekstrand-Hammarström B, Hong J, Davoodpour P, Sandholm K, Ekdahl KN, Bucht A, et al. TiO2 nanoparticles tested in a novel screening whole human blood model of toxicity trigger adverse activation of the kallikrein system at low concentrations. Biomaterials. 2015;51:58–68. https://doi.org/10.1016/j.biomaterials.2015.01.031.
Jiang L, Li Y, Li Y, Guo C, Yu Y, Zou Y, et al. Silica nanoparticles induced the pre-thrombotic state in rats via activation of coagulation factor XII and the JNK-NF-κB/AP-1 pathway. Toxicol Res. 2015;4(6):1453–64. https://doi.org/10.1039/c5tx00118h.
Nabeshi H, Yoshikawa T, Matsuyama K, Nakazato Y, Arimori A, Isobe M, et al. Amorphous nanosilicas induce consumptive coagulopathy after systemic exposure. Nanotechnology. 2012;23(4): 045101. https://doi.org/10.1088/0957-4484/23/4/045101.
•• Hao F, Liu QS, Chen X, Zhao X, Zhou Q, Liao C, et al. Exploring the heterogeneity of nanoparticles in their interactions with plasma coagulation factor XII. ACS Nano. 2019;13(2):1990–2003. https://doi.org/10.1021/acsnano.8b08471. This article shows the distinct interaction of plasma KKS with nanoparticles with different physiochemical characteristics.
Lira AL, Mina N, Bonturi CR, Nogueira RS, Torquato RJS, Oliva MLV, et al. Anionic ultrasmall gold nanoparticles bind to coagulation factors and disturb normal hemostatic balance. Chem Res Toxicol. 2022;35(9):1558–69. https://doi.org/10.1021/acs.chemrestox.2c00190.
•• Long Y, Zhao X, Clermont A, Zhou Q, Liu Q, Feener EP, et al. Negatively charged silver nanoparticles cause retinal vascular permeability by activating plasma contact system and disrupting adherens junction. Nanotoxicology. 2016;10(4):501–11. https://doi.org/10.3109/17435390.2015.1088589. This article reveals for the first time that silver nanoparticles can induce an increase in retinal vascular permeability by activating plasma KKS.
Xia S, Li J, Zu M, Li J, Liu J, Bai X, et al. Small size fullerenol nanoparticles inhibit thrombosis and blood coagulation through inhibiting activities of thrombin and FXa. Nanomed-Nanotechnol. 2018;14(3):929–39. https://doi.org/10.1016/j.nano.2017.12.013.
Robertson S, Miller MR. Ambient air pollution and thrombosis. Part Fibre Toxicol. 2018;15(1):1. https://doi.org/10.1186/s12989-017-0237-x.
Ghio AJ, Hall A, Bassett MA, Cascio WE, Devlin RB. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhalation Toxicol. 2003;15(14):1465–78. https://doi.org/10.1080/08958370390249111.
Møller P, Mikkelsen L, Vesterdal LK, Folkmann JK, Forchhammer L, Roursgaard M, et al. Hazard identification of particulate matter on vasomotor dysfunction and progression of atherosclerosis. Crit Rev Toxicol. 2011;41(4):339–68. https://doi.org/10.3109/10408444.2010.533152.
Rao X, Zhong J, Brook RD, Rajagopalan S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid Redox Signal. 2018;28(9):797–818. https://doi.org/10.1089/ars.2017.7394.
Silva TD, Alves C, Oliveira H, Duarte IF. Metabolic dysregulations underlying the pulmonary toxicity of atmospheric fine particulate matter: focus on energy-producing pathways and lipid metabolism. Air Qual Atmos Health. 2022;15(11):2051–65. https://doi.org/10.1007/s11869-022-01236-6.
Tamagawa E, Bai N, Morimoto K, Gray C, Mui T, Yatera K, et al. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L79-85. https://doi.org/10.1152/ajplung.00048.2007.
Jin X, Yu H, Wang B, Sun Z, Zhang Z, Liu QS, et al. Airborne particulate matters induce thrombopoiesis from megakaryocytes through regulating mitochondrial oxidative phosphorylation. Part Fibre Toxicol. 2021;18(1):19. https://doi.org/10.1186/s12989-021-00411-4.
•• Jin X, Ma Q, Sun Z, Yang X, Zhou Q, Qu G, et al. Airborne fine particles induce hematological effects through regulating the crosstalk of the kallikrein-kinin, complement, and coagulation systems. Environ Sci Technol. 2019;53(5):2840–51. https://doi.org/10.1021/acs.est.8b05817. This research for the first time reveals that airborne fine particulate matter induces the crosstalk among plasma zymogen systems.
•• Zhang Y, Pei Y, Liu QS, Gao Y, Min K, Chen Z, et al. Tracing the plasma kallikrein-kinin system-activating component in the atmospheric particulate matter with different origins. J Hazard Mater. 2023;458:132044. https://doi.org/10.1016/j.jhazmat.2023.132044. This study is the first to fractionate atmospheric fine particulate matter to trace the bioactive components for KKS activation, and reveals that oxidized carbon black particles are the major contributor.
KilinÇ E, Van Oerle R, Borissoff JI, Oschatz C, Gerlofs-Nijland ME, Janssen NA, et al. Factor XII activation is essential to sustain the procoagulant effects of particulate matter. J Thromb Haemostasis. 2011;9(7):1359–67. https://doi.org/10.1111/j.1538-7836.2011.04280.x.
Wang B, Yan X, Chen F, Yang A, Lu Y, Wu Y. Plasma kallikrein contributes to ambient particulate matter-induced lung injury. Biochem Biophys Res Commun. 2019;518(3):409–15. https://doi.org/10.1016/j.bbrc.2019.07.060.
Simak J, De Paoli S. The effects of nanomaterials on blood coagulation in hemostasis and thrombosis. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(5). https://doi.org/10.1002/wnan.1448.
Chen L, Glass JJ, De Rose R, Sperling C, Kent SJ, Houston ZH, et al. Influence of charge on hemocompatibility and immunoreactivity of polymeric nanoparticles. ACS Appl Bio Mater. 2018;1(3):756–67. https://doi.org/10.1021/acsabm.8b00220.
Chen Z, Li F, Liu C, Guan J, Hu X, Du G, et al. Blood clot initiation by mesoporous silica nanoparticles: dependence on pore size or particle size? J Mater Chem B. 2016;4(44):7146–54. https://doi.org/10.1039/c6tb01946c.
Tran HDN, Akther F, Xu ZP, Ta HT. Chapter 6 - Effects of nanoparticles on the blood coagulation system (nanoparticle interface with the blood coagulation system). In: Denizli A, Nguyen TA, Rajan M, Alam MF, Rahman K, editors. Nanotechnology for Hematology, Blood Transfusion, and Artificial Blood. Elsevier; 2022.
Del Turco S, Ciofani G, Cappello V, Parlanti P, Gemmi M, Caselli C, et al. Effects of cerium oxide nanoparticles on hemostasis: coagulation, platelets, and vascular endothelial cells. J Biomed Mater Res, Part A. 2019;107(7):1551–62. https://doi.org/10.1002/jbm.a.36669.
Hao F, Geng F, Zhao X, Liu R, Liu QS, Zhou Q, et al. Chirality of gold nanocluster affects its interaction with coagulation factor XII. NanoImpact. 2021;22: 100321. https://doi.org/10.1016/j.impact.2021.100321.
Khoury LR, Kost J, Enden G. Effects of surface coating on nanoparticle-protein adsorption selectivity. Regener Eng Transl Med. 2018;4(2):62–74. https://doi.org/10.1007/s40883-018-0049-z.
Liu QS, Zhang Y, Sun Z, Gao Y, Zhou Q, Jiang G. A high-throughput assay for screening the abilities of per- and polyfluoroalkyl substances in inducing plasma kallikrein-like activity. Ecotoxicol Environ Saf. 2022;234: 113381. https://doi.org/10.1016/j.ecoenv.2022.113381.
Simberg D, Zhang WM, Merkulov S, McCrae K, Park JH, Sailor MJ, et al. Contact activation of kallikrein-kinin system by superparamagnetic iron oxide nanoparticles in vitro and in vivo. J Control Release. 2009;140(3):301–5. https://doi.org/10.1016/j.jconrel.2009.05.035.
Fan X, Wang S, Fang Y, Li P, Zhou W, Wang Z, et al. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater Sci Eng Mat Sci Eng C. 2020;109: 110649. https://doi.org/10.1016/j.msec.2020.110649.
Duan J, Liang S, Yu Y, Li Y, Wang L, Wu Z, et al. Inflammation-coagulation response and thrombotic effects induced by silica nanoparticles in zebrafish embryos. Nanotoxicology. 2018;12(5):470–84. https://doi.org/10.1080/17435390.2018.1461267.
Visser M, Heitmeier S, Ten Cate H, Spronk HMH. Role of factor XIa and plasma kallikrein in arterial and venous thrombosis. Thromb Haemost. 2020;120(6):883–993. https://doi.org/10.1055/s-0040-1710013.
Bird JE, Smith PL, Wang X, Schumacher WA, Barbera F, Revelli JP, et al. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher trait. Thromb Haemost. 2012;107(6):1141–50. https://doi.org/10.1160/th-11-10-0682.
Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87(4):774–6.
Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146(6):882–93. https://doi.org/10.1038/sj.bjp.0706386.
Nemmar A, Beegam S, Yuvaraju P, Yasin J, Tariq S, Attoub S, et al. Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part Fibre Toxicol. 2016;13(1):22. https://doi.org/10.1186/s12989-016-0132-x.
Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35(6):881–900. https://doi.org/10.1016/s1357-2725(02)00271-6.
Hofman ZLM, de Maat S, Suffritti C, Zanichelli A, van Doorn C, Sebastian SAE, et al. Cleaved kininogen as a biomarker for bradykinin release in hereditary angioedema. J Allergy Clin Immunol. 2017;140(6):1700-3.e8. https://doi.org/10.1016/j.jaci.2017.07.012.
Guerra-Ojeda S, Marchio P, Rueda C, Suarez A, Garcia H, Victor VM, et al. Cerium dioxide nanoparticles modulate antioxidant defences and change vascular response in the human saphenous vein. Free Radic Biol Med. 2022;193(Pt 2):694–701. https://doi.org/10.1016/j.freeradbiomed.2022.11.012.
Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99(1):6–38. https://doi.org/10.1254/jphs.srj05001x.
Govers-Riemslag JWP, Smid M, Cooper JA, Bauer KA, Rosenberg RD, Hack CE, et al. The plasma kallikrein–kinin system and risk of cardiovascular disease in men. J Thromb Haemost. 2007;5(9):1896–903. https://doi.org/10.1111/j.1538-7836.2007.02687.x.
Marcondes S, Antunes E. The plasma and tissue kininogen-kallikrein-kinin system: role in the cardiovascular system. Curr Med Chem Cardiovasc Hematol Agents. 2005;3(1):33–44. https://doi.org/10.2174/1568016052773351.
Cockcroft JR, Chowienczyk PJ, Brett SE, Bender N, Ritter JM. Inhibition of bradykinin-induced vasodilation in human forearm vasculature by icatibant, a potent B2-receptor antagonist. Br J Clin Pharmacol. 1994;38(4):317–21. https://doi.org/10.1111/j.1365-2125.1994.tb04360.x.
Witherow FN, Helmy A, Webb DJ, Fox KAA, Newby DE. Bradykinin contributes to the vasodilator effects of chronic angiotensin-converting enzyme inhibition in patients with heart failure. Circulation. 2001;104(18):2177–81. https://doi.org/10.1161/hc4301.098252.
van Montfoort ML, Meijers JCM. Recent insights into the role of the contact pathway in thrombo-inflammatory disorders. Hematology. 2014;2014(1):60–5. https://doi.org/10.1182/asheducation.V2014.1.60.3882400.
Kolte D, Shariat-Madar Z. Plasma kallikrein inhibitors in cardiovascular disease: an innovative therapeutic approach. Cardiol Rev. 2016;24(3):99–109. https://doi.org/10.1097/crd.0000000000000069.
Chen Y, Qin Z, Wang Y, Li X, Zheng Y, Liu Y. Role of inflammation in vascular disease-related perivascular adipose tissue dysfunction. Front Endocrinol (Lausanne). 2021;12: 710842. https://doi.org/10.3389/fendo.2021.710842.
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–32. https://doi.org/10.1038/s41591-019-0675-0.
Supreeya S, Amandeep G, Yulia G, Roman Z. Metabolic syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. https://doi.org/10.1155/2021/1497449.
Tomita H, Sanford RB, Smithies O, Kakoki M. The kallikrein–kinin system in diabetic nephropathy. Kidney Int. 2012;81(8):733–44. https://doi.org/10.1038/ki.2011.499.
Härma MA, Dahlström EH, Sandholm N, Forsblom C, Groop PH, Lehto M. Decreased plasma kallikrein activity is associated with reduced kidney function in individuals with type 1 diabetes. Diabetologia. 2020;63(7):1349–54. https://doi.org/10.1007/s00125-020-05144-1.
Maltais I, Bachvarova M, Maheux P, Perron P, Marceau F, Bachvarov D. Bradykinin B2 receptor gene polymorphism is associated with altered urinary albumin/creatinine values in diabetic patients. Can J Physiol Pharmacol. 2002;80(4):323–7. https://doi.org/10.1139/y02-036.
Sharma JN, Al-Sherif GJ. Pharmacologic targets and prototype therapeutics in the kallikrein-kinin system: bradykinin receptor agonists or antagonists. The Scientific World J. 2006;6: 298486. https://doi.org/10.1100/tsw.2006.226.
Sidorenkov G, Navis G. Safety of ACE inhibitor therapies in patients with chronic kidney disease. Expert Opin Drug Saf. 2014;13(10):1383–95. https://doi.org/10.1517/14740338.2014.951328.
Kakoki M, Takahashi N, Jennette JC, Smithies O. Diabetic nephropathy is markedly enhanced in mice lacking the bradykinin B2 receptor. Proc Natl Acad Sci U S A. 2004;101(36):13302–5. https://doi.org/10.1073/pnas.0405449101.
Kakoki M, Hirata Y, Hayakawa H, Suzuki E, Nagata D, Nishimatsu H, et al. Effects of vasodilatory antihypertensive agents on endothelial dysfunction in rats with ischemic acute renal failure. Hypertens Res. 2000;23(5):527–33. https://doi.org/10.1291/hypres.23.527.
Kakoki M, McGarrah RW, Kim HS, Smithies O. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2007;104(18):7576–81. https://doi.org/10.1073/pnas.0701617104.
Zhu J, Wang H, Chen J, Wei W. Inhibition of plasma kallikrein–kinin system to alleviate renal injury and arthritis symptoms in rats with adjuvant-induced arthritis. Immunopharmacol Immunotoxicol. 2018;40(2):134–48. https://doi.org/10.1080/08923973.2017.1418883.
Srinivasan S, Kryza T, Batra J, Clements J. Remodelling of the tumour microenvironment by the kallikrein-related peptidases. Nat Rev Cancer. 2022;22(4):223–38. https://doi.org/10.1038/s41568-021-00436-z.
Parenti A, Morbidelli L, Ledda F, Granger HJ, Ziche M. The bradykinin/B1 receptor promotes angiogenesis by up-regulation of endogenous FGF-2 in endothelium via the nitric oxide synthase pathway. Faseb j. 2001;15(8):1487–9.
Ishihara K, Hayash I, Yamashina S, Majima M. A potential role of bradykinin in angiogenesis and growth of S-180 mouse tumors. Jpn J Pharmacol. 2001;87(4):318–26. https://doi.org/10.1254/jjp.87.318.
Karnaukhova E. C1-inhibitor: structure, functional diversity and therapeutic development. Curr Med Chem. 2022;29(3):467–88. https://doi.org/10.2174/0929867328666210804085636.
Jerabek-Willemsen M, André T, Wanner R, Roth HM, Duhr S, Baaske P, et al. MicroScale thermophoresis: interaction analysis and beyond. J Mol Struct. 2014;1077:101–13. https://doi.org/10.1016/j.molstruc.2014.03.009.
Branchford BR. Flood VH. 51 - bleeding and thrombosis. In: Kliegman RM, Toth H, Bordini BJ, Basel D, editors. Nelson pediatric symptom-based diagnosis: common diseases and their mimics. 2nd ed. Philadelphia: Elsevier; 2023.
Tokutake T, Baba H, Shimada Y, Takeda W, Sato K, Hiroshima Y, et al. Exogenous magnesium chloride reduces the activated partial thromboplastin times of lupus anticoagulant-positive patients. PLoS ONE. 2016;11(6): e0157835. https://doi.org/10.1371/journal.pone.0157835.
Funding
This study was financially supported by the National Key R&D Program of China (2023YFC3706603) and the National Natural Science Foundation of China (22176203, 22176204, 22193053).
Author information
Authors and Affiliations
Contributions
YR.G.: Conceptualization, Investigation, Methodology, Visualization, Writing original draft; Writing review & editing; YZ.Z.: Investigation, Writing review & editing; ZW.L.: Investigation, Writing review & editing; QS.L.: Supervision, Writing review & editing; QF.Z.: Conceptualization, Resources, Funding acquisition, Supervision, Writing review & editing; GB.J.: Resources, Project administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Zhang, Y., Li, Z. et al. The Plasma Kallikrein-Kinin System: A Hematological Target for Environmental Contaminants. Curr Pollution Rep (2024). https://doi.org/10.1007/s40726-024-00308-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40726-024-00308-8