Abstract
Purpose of Review
Harsher abiotic conditions are projected for many woodland areas, especially in already arid and semi-arid climates such as the Southwestern USA. Stomatal regulation of their aperture is one of the ways plants cope with drought. Interestingly, the dominant species in the Southwest USA, like in many other ecosystems, have different stomatal behaviors to regulate water loss ranging from isohydric (e.g., piñon pine) to anisohydric (e.g., juniper) conditions suggesting a possible niche separation or different but comparable strategies of coping with stress. The relatively isohydric piñon pine is usually presumed to be more sensitive to drought or less desiccation tolerant compared to the anisohydric juniper although both species close their stomata under drought to avoid hydraulic failure, and the mortality of one species (mostly piñon) over the other in the recent droughts can be attributed to insect outbreaks rather than drought sensitivity alone. Furthermore, no clear evidence exists demonstrating that iso- or anisohydric strategy increases water use efficiency over the other consistently. How these different stomatal regulatory tactics enable woody species to withstand harsh abiotic conditions remains a subject of inquiry to be covered in this review.
Recent Findings
This contribution reviews and explores the use of simplified stomatal optimization theories to assess how photosynthesis and transpiration respond to warming (H), drought (D), and combined warming and drought (H+D) for isohydric and anisohydric woody plants experiencing the same abiotic stressors. It sheds light on how simplified stomatal optimization theories can separate between photosynthetic and hydraulic acclimation due to abiotic stressors and how the interactive effects of H+D versus H or D alone can be incorporated into future climate models.
Summary
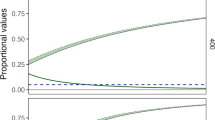
The work here demonstrates how field data can be bridged to simplified optimality principles so as to explore the effect of future changes in temperature and in soil water content on the acclimation of tree species with distinct water use strategies. The results show that the deviations between measurements and predictions from the simplified optimality principle can explain different species’ acclimation behaviors.





Similar content being viewed by others
Data Availability
The datasets generated for this study are available upon request to the corresponding author.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Liu H, Park Williams A, Allen CD, Guo D, Wu X, Anenkhonov OA, Liang E, Sandanov DV, Yin Y, Qi Z, et al. Rapid warming accelerates tree growth decline in semi-arid forests of inner Asia. Glob Chang Biol. 2013;19(8):2500–10.
Park WA, Allen CD, Macalady AK, Griffin D, Woodhouse CA, Meko DM, Swetnam TW, Rauscher SA, Seager R, Grissino-Mayer HD, et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat Clim Chang. 2013;3(3):292–7.
Giannakopoulos C, Hadjinicolaou P, Kostopoulou E, Varotsos KV, Zerefos C. Precipitation and temperature regime over Cyprus as a result of global climate change. Adv Geosci. 2010;23:17–24. https://doi.org/10.5194/adgeo-23-17-2010.
Vogel MM, Orth R, Cheruy F, Hagemann S, Lorenz R, Hurk BJ, Seneviratne SI. Regional amplification of projected changes in extreme temperatures strongly controlled by soil moisture-temperature feedbacks. Geophys Res Lett. 2017;44(3):1511–9.
Givnish TJ. Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol. 1987;106:131–60.
Pittermann J, Stuart SA, Dawson TE, Moreau A. Cenozoic climate change shaped the evolutionary ecophysiology of the Cupressaceae conifers. Proc Natl Acad Sci. 2012;109(24):9647–52.
Johnson HB. Plant pubescence: an ecological perspective. Bot Rev. 1975;41:233–58.
Hacke UG, Spicer R, Schreiber SG, Plavcová L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2017;40(6):831–45.
Olson ME, Anfodillo T, Gleason SM, McCulloh KA. Tip-to-base xylem conduit widening as an adaptation: causes, consequences, and empirical priorities. New Phytol. 2021;229(4):1877–93.
Rodríguez-Ramírez EC, Ferrero ME, Acevedo-Vega I, Crispin-DelaCruz DB, Ticse-Otarola G, Requena-Rojas EJ. Plastic adjustments in xylem vessel traits to drought events in three Cedrela species from Peruvian Tropical Andean forests. Scientific Reports. 2022;12(1):21112.
Martin RE, Asner GP, Bentley LP, Shenkin A, Salinas N, Huaypar KQ, Pillco MM, Ccori Álvarez FD, Enquist BJ, Diaz S, et al. Covariance of sun and shade leaf traits along a tropical forest elevation gradient. Front Plant Sci. 2020;10:1810.
Givnish TJ. Adaptation to sun and shade: a whole-plant perspective. Funct Plant Biol. 1988;15(2):63–92.
Tardieu F, Simonneau T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot. 1998;419–432.
Stocker O. Die abhängigkeit der transpiration von den umweltfaktoren. 1956;436–488.
Domec J-C, Johnson DM. Does homeostasis or disturbance of homeostasis in minimum leaf water potential explain the isohydric versus anisohydric behavior of Vitis vinifera L. cultivars? Tree Physiol. 2012;32(3):245–248.
Meinzer FC, Woodruff DR, Marias DE, Smith DD, McCulloh KA, Howard AR, Magedman AL. Mapping ‘hydroscapes’ along the iso-to anisohydric continuum of stomatal regulation of plant water status. Ecol Lett. 2016;19(11):1343–52.
• Martínez-Vilalta J, Garcia-Forner N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017;40(6):962–976. This review explains the difference between isohydric and anisohydric behavior and shows the existence of a continuum for this behavior.
Adams HD, Zeppel MJ, Anderegg WR, Hartmann H, Landhäusser SM, Tissue DT, Huxman TE, Hudson PJ, Franz TE, Allen CD, et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nature Ecol Evol. 2017;1(9):1285–91.
Parolari AJ, Katul GG, Porporato A. An ecohydrological perspective on drought-induced forest mortality. J Geophys Res Biogeosci. 2014;119(5):965–81.
Attia Z, Domec J-C, Oren R, Way DA, Moshelion M. Growth and physiological responses of isohydric and anisohydric poplars to drought. J Exp Bot. 2015;66(14):4373–81.
Yi K, Maxwell JT, Wenzel MK, Roman DT, Sauer PE, Phillips RP, Novick KA. Linking variation in intrinsic water-use efficiency to isohydricity: a comparison at multiple spatiotemporal scales. New Phytol. 2019;221(1):195–208.
Plaut JA, Yepez EA, Hill J, Pangle R, Sperry JS, Pockman WT, Mcdowell NG. Hydraulic limits preceding mortality in a piñon-juniper woodland under experimental drought. Plant Cell Environ. 2012;35(9):1601–17.
Limousin J-M, Bickford CP, Dickman LT, Pangle RE, Hudson PJ, Boutz AL, Gehres N, Osuna JL, Pockman WT, McDowell NG. Regulation and acclimation of leaf gas exchange in a piñon-juniper woodland exposed to three different precipitation regimes. Plant Cell Environ. 2013;36(10):1812–25.
McDowell NG, Fisher RA, Xu C, Domec J-C, Hölttä T, Mackay DS, Sperry JS, Boutz A, Dickman L, Gehres N, et al. Evaluating theories of drought-induced vegetation mortality using a multimodel-experiment framework. New Phytol. 2013;200(2):304–21.
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 2008;178(4):719–39.
Hoffmann WA, Marchin RM, Abit P, Lau OL. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Glob Chang Biol. 2011;17(8):2731–42.
Garcia-Forner N, Adams HD, Sevanto S, Collins AD, Dickman LT, Hudson PJ, Zeppel MJ, Jenkins MW, Powers H, Martínez-Vilalta J, et al. Responses of two semiarid conifer tree species to reduced precipitation and warming reveal new perspectives for stomatal regulation. Plant Cell Environ. 2016;39(1):38-49.
Pappas C, Matheny AM, Baltzer JL, Barr AG, Black TA, Bohrer G, Detto M, Maillet J, Roy A, Sonnentag O, et al. Boreal tree hydrodynamics: asynchronous, diverging, yet complementary. Tree Physiol. 2018;38(7):953–64.
Fisher RA, Williams M, Do Vale RL, Da Costa AL, Meir P. Evidence from Amazonian forests is consistent with isohydric control of leaf water potential. Plant Cell Environ. 2006;29(2):151–65.
Klein T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct Ecol. 2014;28(6):1313–20.
Benson MC, Miniat CF, Oishi AC, Denham SO, Domec J-C, Johnson DM, Missik JE, Phillips RP, Wood JD, Novick KA. The xylem of anisohydric Quercus alba L. is more vulnerable to embolism than isohydric codominants. Plant Cell Environ. 2022;45(2):329–346.
Collins MJ, Fuentes S, Barlow EW. Partial rootzone drying and deficit irrigation increase stomatal sensitivity to vapour pressure deficit in anisohydric grapevines. Funct Plant Biol. 2010;37(2):128–38.
Romero-Trigueros C, Gambín JMB, Nortes Tortosa PA, Cabañero JJA, Nicolás EN. Isohydricity of two different citrus species under deficit irrigation and reclaimed water conditions. Plants. 2021;10(10):2121.
Schultz HR. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003;26(8):1393–1405.
Johnson DM, Katul G, Domec J-C. Catastrophic hydraulic failure and tipping points in plants. Plant Cell Environ. 2022.
Zeppel MJ, Lewis JD, Chaszar B, Smith RA, Medlyn BE, Huxman TE, Tissue DT. Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytol. 2012;193(4):929–38.
•• Grossiord C, Sevanto S, Borrego I, Chan AM, Collins AD, Dickman LT, Hudson PJ, McBranch N, Michaletz ST, Pockman WT, et al. Tree water dynamics in a drying and warming world. Plant Cell Environ. 2017;40(9):1861–1873. This article provides a detailed explanation for the experiment and data used to generate the results of the case study.
Givnish TJ, Vermeij GJ. Sizes and shapes of liane leaves. Am Nat. 1976;110(975):743–78.
Mäkelä A, Berninger F, Hari P. Optimal control of gas exchange during drought: theoretical analysis. Ann Bot. 1996;77(5):461–8.
•• Cowan I, Troughton J. The relative role of stomata in transpiration and assimilation. Planta. 1971;97(4):325–336. This manuscript introduces the formulation of stomatal optimality principle in plant physiology necessary for the development of the approach used in this study.
Papert S. Mindstorms: computers, children, and powerful ideas. 1980;230.
Bardi U. Mind sized world models. Sustainability. 2013;5(3):896–911.
Franklin O, Harrison SP, Dewar R, Farrior CE, Brännström Å, Dieckmann U, Pietsch S, Falster D, Cramer W, Loreau M, et al. Organizing principles for vegetation dynamics. Nature Plants. 2020;6(5):444–53.
Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 2007;30(3):258–70.
Buckley TN. The control of stomata by water balance. New Phytol. 2005;168(2):275–92.
Campbell GS, Norman JM. An introduction to environmental biophysics. 2000.
• Damour G, Simonneau T, Cochard H, Urban L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 2010;33(9):1419–1438. This article provides an overview of the different stomatal conductance models.
Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33(1):317–45.
• Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424(6951):901–908. This paper highlights the role of stomata and intercellular CO\({}_2\)in regulating climate models.
Hallé F. Jones, hg—Plants and microclimate. A quantitative approach to environmental plant physiology. Cambridge university press, Cambridge, 1983. Revue d’Écologie (La Terre et La Vie). 1984;39(1):123–123.
Meidner H, Zeiger E, Farquhar G, Cowan I. Three hundred years of research into stomata. Stomatal Function. 1987;7–27.
Schulze E-D, Kelliher FM, Körner C, Lloyd J, Leuning R. Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: a global ecology scaling exercise. Annu Rev Ecol Syst. 1994;25(1):629–62.
Darwin F. Ix. Observations on stomata. Philos Trans R Soc Lond B Containing Papers Biol Char. 1898;190:531–621.
Scarth GW. Stomatal movement: its regulation and regulatory role a review. Protoplasma. 1927;2(1):498–511.
Bowen IS. The ratio of heat losses by conduction and by evaporation from any water surface. Phys Rev. 1926;27(6):779.
Penman HL. Natural evaporation from open water, bare soil and grass. Proc R Soc Lond A Math Phys Sci. 1948;193(1032):120–45.
Monteith JL. Evaporation and environment. In: Symposia of the Society for Experimental Biology. Cambridge: University Press (CUP) Cambridge; 1965. vol. 19, p. 205–234.
Jarvis P. The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Philos Trans R Soc Lond B Biol Sci. 1976;273(927):593–610.
Collatz GJ, Ball JT, Grivet C, Berry JA. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: a model that includes a laminar boundary layer. Agric Forest Meteorol. 1991;54(2):107–36. https://doi.org/10.1016/0168-1923(91)90002-8.
Leuning R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ. 1995;18(4):339–55.
Ball JT, Woodrow IE, Berry JA. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Progress in Photosynthesis Research: Volume 4 Proceedings of the VIIth International Congress on Photosynthesis Providence, Rhode Island, USA, August 10–15, 1986. Springer 1987. p. 221–224.
Sellers P, Bounoua L, Collatz G, Randall D, Dazlich D, Los S, Berry J, Fung I, Tucker C, Field C, et al. Comparison of radiative and physiological effects of doubled atmospheric CO2 on climate. Science. 1996;271(5254):1402–6.
• Oren R, Sperry J, Katul G, Pataki D, Ewers B, Phillips N, Schäfer K. Survey and synthesis of intra-and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999;22(12):1515–1526. This work demonstrates the universal role of stomata in response to vapor pressure deficit from different set of data.
Sperry J, Hacke U, Oren R, Comstock J. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002;25(2):251–63.
Liu Y, Kumar M, Katul GG, Feng X, Konings AG. Plant hydraulics accentuates the effect of atmospheric moisture stress on transpiration. Nat Clim Chang. 2020;10(7):691–5.
Cowan I. Stomatal behaviour and environment. In: Advances in Botanical Research. Elsevier, ??? 1978. vol. 4, p. 117–228
Cowan IR, GD F. Stomatal function in relation to leaf metabolism and environment. 1977.
Hari P, Mäkelä A, Korpilahti E, Holmberg M. Optimal control of gas exchange. Tree Physiol. 1986;2(1-2-3):169–175.
Berninger F, Hari P. Optimal regulation of gas exchange: evidence from field data. Ann Bot. 1993;71(2):135–40.
Hari P, Mäkelä A, Pohja T. Surprising implications of the optimality hypothesis of stomatal regulation gain support in a field test. Funct Plant Biol. 2000;27(1):77–80.
Arneth A, Lloyd J, Santrckova H, Bird M, Grigoryev S, Kalaschnikov Y, Gleixner G, Schulze E-D. Response of central Siberian scots pine to soil water deficit and long-term trends in atmospheric co2 concentration. Glob Biogeochem Cycles. 2002;16(1):5–1.
Konrad W, Roth-Nebelsick A, Grein M. Modelling of stomatal density response to atmospheric CO2. J Theor Biol. 2008;253(4):638–58.
Katul GG, Palmroth S, Oren R. Leaf stomatal responses to vapour pressure deficit under current and CO2-enriched atmosphere explained by the economics of gas exchange. Plant Cell Environ. 2009;32(8):968–79.
Medlyn BE, Duursma RA, Eamus D, Ellsworth DS, Prentice IC, Barton CV, Crous KY, De Angelis P, Freeman M Wingate L. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob Chang Biol. 2011;17(6):2134–2144.
Katul G, Manzoni S, Palmroth S, Oren R. A stomatal optimization theory to describe the effects of atmospheric CO2 on leaf photosynthesis and transpiration. Ann Bot. 2010;105(3):431–42.
Katul GG, Oren R, Manzoni S, Higgins C, Parlange MB. Evapotranspiration: a process driving mass transport and energy exchange in the soil-plant-atmosphere-climate system. Rev Geophys. 2012;50(3).
Tuzet A, Perrier A, Leuning R. A coupled model of stomatal conductance, photosynthesis and transpiration. Plant Cell Environ. 2003;26(7):1097–116.
Lai C-T, Katul G, Oren R, Ellsworth D, Schäfer K. Modeling CO2 and water vapor turbulent flux distributions within a forest canopy. J Geophys Res Atmos. 2000;105(D21):26333–51.
Cowan I, et al. Economics of carbon fixation in higher plants. Economics of carbon fixation in higher plants. 1986. p. 133–170.
Manzoni S, Vico G, Palmroth S, Porporato A, Katul G. Optimization of stomatal conductance for maximum carbon gain under dynamic soil moisture. Adv Water Resour. 2013;62:90–105.
•• Mrad A, Sevanto S, Domec J-C, Liu Y, Nakad M, Katul G. A dynamic optimality principle for water use strategies explains isohydric to anisohydric plant responses to drought. Front Forest Global Change. 2019;2:49. This study provides a derivation for the dynamic optimality principles and shows the connections between the Hamiltonian and physiological fluxes.
Feng X, Lu Y, Jiang M, Katul G, Manzoni S, Mrad A, Vico G. Instantaneous stomatal optimization results in suboptimal carbon gain due to legacy effects. Plant Cell Environ. 2022
Manzoni S, Vico G, Katul G, Fay PA, Polley W, Palmroth S, Porporato A. Optimizing stomatal conductance for maximum carbon gain under water stress: a meta-analysis across plant functional types and climates. Funct Ecol. 2011;25(3):456–67.
Parlange J-Y, Waggoner PE. Stomatal dimensions and resistance to diffusion. Plant Physiol. 1970;46(2):337–42.
Witelski T, Bowen M. Variational Principles. In: Witelski T, Bowen M, editors. Methods of mathematical modelling: continuous systems and differential equations. Springer Undergraduate Mathematics Series. Springer: Cham; 2015. p. 47–83. https://doi.org/10.1007/978-3-319-23042-9_3.
Fites J, Teskey R. CO2 and water vapor exchange of Pinus taeda in relation to stomatal behavior: test of an optimization hypothesis. Can J For Res. 1988;18(2):150–7.
•• Farquhar GD, Caemmerer Sv, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149(1):78–90. This study covers the formulation of the biochemical demand for CO\(_2\)by C3 plants, a necessary step for the conceptualization of stomatal optimization.
Qiu R, Katul GG. Maximizing leaf carbon gain in varying saline conditions: an optimization model with dynamic mesophyll conductance. Plant J. 2020;101(3):543–54.
Volpe V, Manzoni S, Marani M, Katul G. Leaf conductance and carbon gain under salt-stressed conditions. J Geophys Res Biogeosci. 2011;116(G4).
Mackay D, Ahl D, Ewers B, Samanta S, Gower S, Burrows S. Physiological tradeoffs in the parameterization of a model of canopy transpiration. Adv Water Resour. 2003;26(2):179–94.
Launiainen S, Katul GG, Kolari P, Vesala T, Hari P. Empirical and optimal stomatal controls on leaf and ecosystem level CO2 and H2O exchange rates. Agric Forest Meteorol. 2011;151(12):1672–89.
• Monteith J. A reinterpretation of stomatal responses to humidity. Plant Cell Environ. 1995;18(4):357–364. This manuscript introduces the feed-forward mecahnism of stomata. This mechanism is apparent in the result of stomatal optimization.
Schulze E-D, Lange O, Buschbom U, Kappen L, Evenari M. Stomatal responses to changes in humidity in plants growing in the desert. Planta. 1972;108(3):259–70.
Franks P, Cowan I, Farquhar G. The apparent feedforward response of stomata to air vapour pressure deficit: information revealed by different experimental procedures with two rainforest trees. Plant Cell Environ. 1997;20(1):142–5.
Lhomme J-P. Stomatal control of transpiration: examination of the Jarvis-type representation of canopy resistance in relation to humidity. Water Resour Res. 2001;37(3):689–99.
Turner NC, Schulze E-D, Gollan T. The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content. Oecologia. 1984;63(3):338–42.
Macfarlane C, White D, Adams M. The apparent feed-forward response to vapour pressure deficit of stomata in droughted, field-grown Eucalyptus globulus Labill. Plant Cell Environ. 2004;27(10):1268–80.
Duursma RA, Barton CV, Lin Y-S, Medlyn BE, Eamus D, Tissue DT, Ellsworth DS, McMurtrie RE. The peaked response of transpiration rate to vapour pressure deficit in field conditions can be explained by the temperature optimum of photosynthesis. Agric Foret Meteorol. 2014;189:2–10.
Vico G, Manzoni S, Palmroth S, Weih M, Katul G. A perspective on optimal leaf stomatal conductance under CO2 and light co-limitations. Agric Forest Meteorol. 2013;182:191–9.
Palmroth S, Berninger F, Nikinmaa E, Lloyd J, Pulkkinen P, Hari P. Structural adaptation rather than water conservation was observed in Scots pine over a range of wet to dry climates. Oecologia. 1999;121(3):302–9.
Wong S, Dunin F. Photosynthesis and transpiration of trees in a eucalypt forest stand: CO2, light and humidity responses. Funct Plant Biol. 1987;14(6):619–32.
Katul G, Ellsworth D, Lai C-T. Modelling assimilation and intercellular CO2 from measured conductance: a synthesis of approaches. Plant Cell Environ. 2000;23(12):1313–28.
Zhou S, Yu B, Huang Y, Wang G. Daily underlying water use efficiency for AmeriFlux sites. J Geophys Res Biogeosci. 2015;120(5):887–902.
Nelson JA, Pérez-Priego O, Zhou S, Poyatos R, Zhang Y, Blanken PD, Gimeno TE, Wohlfahrt G, Desai AR, Gioli B, et al. Ecosystem transpiration and evaporation: insights from three water flux partitioning methods across FLUXNET sites. Glob Chang Biol. 2020;26(12):6916–30.
Zhou S, Yu B, Zhang Y, Huang Y, Wang G. Partitioning evapotranspiration based on the concept of underlying water use efficiency. Water Resour Res. 2016;52(2):1160–75.
Perez-Priego O, Katul G, Reichstein M, El-Madany TS, Ahrens B, Carrara A, Scanlon TM, Migliavacca M. Partitioning eddy covariance water flux components using physiological and micrometeorological approaches. J Geophys Res Biogeosci. 2018;123(10):3353–70.
Zahn E, Bou-Zeid E, Good SP, Katul GG, Thomas CK, Ghannam K, Smith JA, Chamecki M, Dias NL, Fuentes JD, et al. Direct partitioning of eddy-covariance water and carbon dioxide fluxes into ground and plant components. Agric Forest Meteorol. 2022;315: 108790.
Mott K, Parkhurst D. Stomatal responses to humidity in air and Helox. Plant Cell Environ. 1991;14(5):509–15.
Siqueira M, Katul G, Porporato A. Onset of water stress, hysteresis in plant conductance, and hydraulic lift: scaling soil water dynamics from millimeters to meters. Water Resour Res. 2008;44(1).
Siqueira M, Katul G, Porporato A. Soil moisture feedbacks on convection triggers: the role of soil-plant hydrodynamics. J Hydrometeorol. 2009;10(1):96–112.
Manoli G, Bonetti S, Domec J-C, Putti M, Katul G, Marani M. Tree root systems competing for soil moisture in a 3D soil-plant model. Adv Water Resour. 2014;66:32–42.
Manoli G, Huang C-W, Bonetti S, Domec J-C, Marani M, Katul G. Competition for light and water in a coupled soil-plant system. Adv Water Resour. 2017;108:216–30.
Sperry JS, Love DM. What plant hydraulics can tell us about responses to climate-change droughts. New Phytol. 2015;207(1):14–27.
Huang CW, Domec JC, Palmroth S, Pockman WT, Litvak ME, Katul GG. Transport in a coordinated soil-root-xylem-phloem leaf system. Adv Water Resour. 2018;119:1–16.
Sperry JS, Venturas MD, Anderegg WR, Mencuccini M, Mackay DS, Wang Y, Love DM. Predicting stomatal responses to the environment from the optimization of photosynthetic gain and hydraulic cost. Plant Cell Environ. 2017;40(6):816–30.
Anderegg WR, Wolf A, Arango-Velez A, Choat B, Chmura DJ, Jansen S, Kolb T, Li S, Meinzer FC, Pita P, et al. Woody plants optimise stomatal behaviour relative to hydraulic risk. Ecol Lett. 2018;21(7):968–77.
Venturas MD, Sperry JS, Hacke UG. Plant xylem hydraulics: what we understand, current research, and future challenges. J Integr Plant Biol. 2017;59(6):356–89.
Dewar R, Mauranen A, Mäkelä A, Hölttä T, Medlyn B, Vesala T. New insights into the covariation of stomatal, mesophyll and hydraulic conductances from optimization models incorporating nonstomatal limitations to photosynthesis. New Phytol. 2018;217(2):571–85.
Flexas J, Scoffoni C, Gago J, Sack L. Leaf mesophyll conductance and leaf hydraulic conductance: an introduction to their measurement and coordination. J Exp Bot. 2013;64(13):3965–81.
Jensen KH, Lee J, Bohr T, Bruus H, Holbrook NM, Zwieniecki MA. Optimality of the Münch mechanism for translocation of sugars in plants. J R Soc Interface. 2011;8(61):1155–65.
Nikinmaa E, Hölttä T, Hari P, Kolari P, Mäkelä A, Sevanto S, Vesala T. Assimilate transport in phloem sets conditions for leaf gas exchange. Plant Cell Environ. 2013;36(3):655–69.
Nikinmaa E, Sievänen R, Hölttä T. Dynamics of leaf gas exchange, xylem and phloem transport, water potential and carbohydrate concentration in a realistic 3-D model tree crown. Ann Bot. 2014;114(4):653–66.
Nakad M, Domec J-C, Sevanto S, Katul G. Radial-axial transport coordination enhances sugar translocation in the phloem vasculature of plants. Plant Physiol. 2022. https://doi.org/10.1093/plphys/kiac231.
Konrad W, Katul G, Roth-Nebelsick A, Jensen KH. Xylem functioning, dysfunction and repair: a physical perspective and implications for phloem transport. Tree Physiol. 2018;39(2):243–61.
Jensen KH, Berg-Sørensen K, Bruus H, Holbrook NM, Liesche J, Schulz A, Zwieniecki MA, Bohr T. Sap flow and sugar transport in plants. Rev Mod Phys. 2016;88(3): 035007.
Prentice IC, Dong N, Gleason SM, Maire V, Wright IJ. Balancing the costs of carbon gain and water transport: testing a new theoretical framework for plant functional ecology. Ecol Lett. 2014;17(1):82–91.
Perri S, Katul GG, Molini A. Xylem-phloem hydraulic coupling explains multiple osmoregulatory responses to salt stress. New Phytol. 2019;224(2):644–62.
Katul G, Leuning R, Oren R. Relationship between plant hydraulic and biochemical properties derived from a steady-state coupled water and carbon transport model. Plant Cell Environ. 2003;26(3):339–50.
Turner NC. Turgor maintenance by osmotic adjustment: a review and evaluation. Adaptation of plants to water and high temperature stress. 1980. p. 87–103.
Meinzer FC, Woodruff DR, Marias DE, McCulloh KA, Sevanto S. Dynamics of leaf water relations components in co-occurring iso- and anisohydric conifer species. Plant Cell Environ. 2014;37(11):2577–86.
Jasechko S, Sharp ZD, Gibson JJ, Birks SJ, Yi Y, Fawcett PJ. Terrestrial water fluxes dominated by transpiration. Nature. 2013;496(7445):347–50.
Griffiths H, Helliker BR. Mesophyll conductance: internal insights of leaf carbon exchange. Plant Cell Environ. 2013;36(4):733–5.
Brodersen CR, McElrone AJ. Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Front Plant Sci. 2013;4:108.
Funding
This work was supported by the US National Science Foundation (NSF-IOS-1754893, NSF-AGS-2028633), the Department of Energy (DE-SC0022072 and DE-SC0023309), the Los Alamos Directed Research and Development Exploratory Research Grant (No. 2020109DR), and the region Nlle. Aquitaine (projects VITIPIN, ANR PHydrauCC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakad, M., Sevanto, S., Domec, JC. et al. Linking the Water and Carbon Economies of Plants in a Drying and Warming Climate. Curr. For. Rep. 9, 383–400 (2023). https://doi.org/10.1007/s40725-023-00202-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-023-00202-4