Abstract
The recently reported laser pyrolytic jetting process enables facile generation of highly porous, free-standing graphene fiber from polyimide film upon the scanning of tightly focused continuous-wave laser. As a follow-up study, we claim that the corresponding laser pyrolytic jetting process is favorable for the fabrication of relevant energy device applications compared to the conventional LIG process in both energy-saving and material-saving perspectives. Moreover, the volume of the pyrolysis product is substantially increased in the case of pyrolytic jetting compared to conventional LIG, resulting in an extended surface area for storing more electric charges. At the same time, material characterization by Raman measurement validates that the exfoliated product from pyrolytic jetting is analogous to the conventional LIG and therefore suitable for supercapacitor application. As a result, supercapacitor created by the pyrolytic jetting allows substantial enhancement in the capacitance compared to the conventional LIG for both with and without manganese oxide layer coating, proposing the potential of pyrolytic jetting for the efficient fabrication of carbon-based energy devices.
Similar content being viewed by others
1 Introduction
The importance of energy storage has become increasingly prominent in recent times [1,2,3,4], owing to the rising adoption of renewable energy in pursuit of carbon neutrality [5, 6]. The intermittent nature of energy production from renewable sources, such as solar and wind [7], necessitates the development of efficient energy storage systems to stabilize power grids, ensuring that the supply of energy remains consistent even when renewable energy production fluctuates. At the same time, rapidly increasing adoption of electric vehicles also has emphasized the importance of energy storage solutions. As traditional combustion engines are replaced by electric-powered vehicles, there is a growing demand for energy storage systems that are safe, reliable, and cost-effective, and can provide the necessary power [8, 9]. Therefore, energy storage has become a critical factor in realizing the full potential of electric mobility. These developments have made it clear that energy storage plays an increasingly significant role in shaping our energy landscape as well as ensuring future energy security [10]. As our society moves towards a more sustainable and low-carbon future [11], the realization of effective and versatile energy storage solutions [12] becomes even more critical.
With the ever-growing need for effective energy storage methods, supercapacitors have emerged as an alternative option to address deficiencies of batteries for specific energy storage applications with their own advantages, such as higher power density, longer cycle life and simpler material compositions [13]. Meanwhile, among various carbon-based materials that are commonly used for supercapacitor electrodes including activated carbon [14,15,16] and carbon-based nanomaterials [17], graphene stands out for its fast charging/discharging capabilities and high specific surface area [18, 19]. Consequently, graphene-based supercapacitors have gained significant attention and are prepared through various methods, including reduced graphene oxide and chemically modified graphene [20]. Among them, laser-induced graphene (LIG) stands out due to its simple fabrication process and porous morphology, making it an attractive candidate for various applications such as in-plane micro-supercapacitors [21, 22], water filters [23], food spoilage detection [24], wearable devices, electronic skin [25], and biosensors [26, 27]. The utilization of LIG in supercapacitors also has shown promising results [28], particularly due to its unique morphology, which is expected to play a decisive role in expanding the surface-area-to-volume ratio for an enhanced performance. From an environmentally conscious perspective, it is crucial to minimize both the energy consumption and material usage required for the fabrication process while simultaneously maximizing the storage capacity. Recently, a phenomenon called pyrolytic jetting has been reported under specific laser conditions [29], where both delamination of the pyrolysis product and radical volume expansion occur simultaneously. This process generates an extremely porous, free-standing fiber-shaped LIG that are possibly beneficial for energy storage applications. In comparison to electrodes affixed to specific substrates, fiber-shaped electrodes exhibit notable advantages as energy devices for wearable applications, particularly in terms of their exceptional flexibility and breathability [30, 31]. Despite its huge potential, there has been limited research on its application in actual energy devices.
In this study, we investigate the potential application of highly porous graphene fibers generated by pyrolytic jetting as supercapacitor, with a particular focus on comparing their performance to that of LIG remaining in a PI matrix. It is firstly confirmed that the pyrolytic jetting process can produce notably expanded graphene fiber compared to the conventional LIG at the same fluence. The resultant volume of the product created by the pyrolytic jetting can be more than an order of magnitude larger than the original volume subject to the laser pyrolysis, suggesting that the morphology of resultant free-standing graphene fiber is suitable for storing more electric charge at the interface. As a result, supercapacitors created on free-standing LIG fiber show considerably larger capacitance compared to the ones based on the conventional LIG. Furthermore, when coated with manganese oxide, both LF-based and LIG-based supercapacitors exhibited significantly improved capacitance, and the performance disparity between the two became even more pronounced. Specifically, the capacitance of LF was measured to be approximately 4.69 times larger than LIG under the same MnO2 coating and laser conditions. Our findings demonstrate that self-standing LF produced through laser pyrolytic jetting offers a significantly larger active surface area compared to conventional LIG formed on substrates, rendering it highly suitable for environmentally-friendly and high-performance energy device fabrication.
2 Result and Discussion
2.1 Generation Conditions and Characteristics of LIG-Fiber
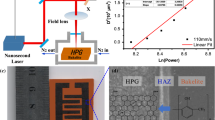
In this study, we investigate the properties and potential applications of carbon fibers fabricated through pyrolytic jetting, a novel laser-based technique that utilizes the continuous-wave laser with a wavelength of 532 nm. As schematically shown in Fig. 1a, the characteristics of the final pyrolysis products can be vastly different according to specific laser conditions: in general, LIG remains embedded in the matrix as reported elsewhere [32], (Fig. 1a, i) while under certain conditions, a different form of free-standing LIG fiber (LF) that has a noticeably increased length is spontaneously ejected from the substrate (Fig. 1a, ii). The unique morphology of the fiber is attributed to the extreme temperature gradients induced by the rapid scanning of a tightly focused laser beam [33]. The resultant carbonization products of the LF have significantly larger surface area due to the enhanced length coupled with highly porous structure, which is expected to be beneficial as electrodes for energy storage devices. Since the generation of LIG in either morphology requires laser scanning procedure, we adopted the unit of energy per unit length (J/m) [34] to compare pyrolytic jetting fibers with conventional LIG from the perspective of the energy required to process a unit length of the scanning length. It should be noted that the laser power and scanning speed should be increased at the same time to maintain the energy per unit length constant. Since the beam spot size is not changing, the energy per unit length also can be considered as laser fluence.
Figure 2a shows the conditions for generating LF in terms of the laser power and the fluence. In the red region, which corresponds to low laser power and scanning speed, laser scanning fails to create any pyrolytic jetting phenomenon due to insufficient temperature gradient and results in LIG that is not separated from the polyimide matrix (Fig. 2b, i). Along the same energy per unit length, (200 J/m) The optimal region for continuous LF generation (Fig. 2b, iii) is found between two unstable regions (Fig. 2b, ii and iv), where the laser power and scanning speed are both within certain threshold ranges. It is observable that the various forms of LIG can be generated even at the same fluence, and it is highly probable from the optical microscopic images that the LF enables more energy-efficient manufacturing of energy devices by providing highly enhanced surface area compared to the conventional LIG within the matrix [35].
Although it is shown from Fig. 2a that the pyrolytic jetting is available over wide range of fluence, it remains uncertain that the microstructures and the constituent material compositions are appropriate for energy device applications. In this regard, energy per unit length is altered within the range that generates LF by changing laser scanning speed at the fixed laser power of 550 mW to investigate their microscopic properties. The Fig. 3a demonstrate that the length of the LF changes according to the fluence, and the overall trend shows that the LFs become longer as the scanning speed increases due to more sudden temperature increase experienced at the scanning front. On the other hand, a higher fluence indicates that more energy is applied per unit length, resulting in increased graphitization that affects electrical and mechanical properties of the resultant product as well [32, 36]. As the electrical conductivity and mechanical compliance of LIG are known to be in a trade-off relationship [37], the fluence should be selected based on the desired physical properties for the specific final application. Slower scanning speed at the fixed laser power, at the same time, increases the thermal diffusion towards lateral direction to result in LF at a larger diameter as shown in Fig. 3b. Despite of these changes in dimensions, highly porous nature of LF remains in every LF as confirmed from the corresponding SEM images in Fig. 3c.
In Fig. 4a, the representative Raman spectra of LF shows a higher degree of graphitization by the growing proportion of graphitic carbons (2D peak) with decreasing defects (D peak) as the applied fluence increases [38, 39]. This tendency is also observed from the lower presence of amorphous carbon (a-C), which is shown as a broad band in between the D and G peaks, over a certain level of energy per unit length values [40]. Together with the intensity ratio of the characteristic peaks, such as ID/IG and Ia−C/IG, the full width at half maximum (FWHM) value of the G peaks provides more information on crystallinity (Fig. 4b). The linear relationship between the narrowing of the G peak with increasing energy per unit length values results from the higher sp2 contents which represents the increase in graphitic ordering within the LF samples [41]. Compared to the commonly fabricated LIG at 125 J/m condition, the LF contains lower defects and higher crystallinity that can promote its electrochemical properties when applied as a supercapacitor [42].
The length and cross-sectional area of the LF that is generated by scanning the same length according to the laser fluence are as follows (Fig. 5a). When these values are multiplied together as shown in Fig. 5b, the resulting volume is highest at the maximum energy per unit length (125 J/m). However, when considering the degree of expansion relative to the initial volume estimated from the remaining matrix, the largest expansion is observed at the minimum energy per unit length. The results suggest that although a large volume expansion is a common phenomenon in the pyrolytic jetting process, pyrolytic jetting conducted at the minimum energy per unit length leads to the highest degree of expansion, indicating the most effective utilization of the available raw material in terms of volume.
2.2 Performance Comparison and Potential as Supercapacitors
The graph (Fig. 6a) demonstrates the correlation between the fluence used in LF production and its corresponding capacitance values. Increasing the fluence results in a greater volume and porosity of the carbon fiber, leading to an expanded electrode-electrolyte interface area. Consequently, this enhances the overall capacitance of the LF. Additionally, Fig. 6b showcases the cyclic voltammetry (CV) curves, highlighting that LF produced under the 125 J/m condition exhibits superior charge storage capability compared to the LIG fabricated with the same condition. Furthermore, Fig. 6c presents the capacitance difference across varying voltage scan rates. Remarkably, LF consistently outperforms LIG regarding capacitance over the entire voltage scan rate range (25~400 mV/s). To minimize inaccuracies in measurements arising from trapped air within the porous structure of the electrodes, a stabilization period of a couple of hours was implemented after immersing the electrodes in the electrolyte prior to conducting every electrochemical performance experiment.
LIG is chemically inert [43] and therefore capable to withstand other chemical processes. We aimed to enhance the performance of supercapacitors further by using manganese oxide (MnO2), which has a high specific capacitance and good cycling stability, via electroplating process on LIG and LF. The coating was carried out using Mn(NO3)2 (25 mM, 50 mL) + NaNO3(100 mM, 50 mL) solution under a potential of 1 V for 120 s using a potentiometer. Figure 7 shows that the performance of the supercapacitor was notably improved by coating with MnO2 compared to the uncoated LIG and LF. When comparing Fig. 6a–c with Fig. 7a–c, it becomes evident that the overall enhancement in supercapacitor performance is significantly more pronounced for LF than for LIG. The disparity in performance between LF and LIG can be attributed to the difference in their coating processes during MnO2 plating. While LIG, generated on the PI film, is coated only on the topmost surface, LF is peeled off in a free-standing fiber form and subsequently coated, allowing for an omnidirectional coating. This omnidirectional coating provides a larger surface area for MnO2, thereby contributing to the enhanced performance of LF.
MnO2-coated LIG and LF performance comparison. a Capacitance comparison of LF and LIG in terms of energy per unit length after the MnO2 coating process. b CV curve of LF and LIG electrode at the voltage scan rate of 100 mV/s. c Capacitance variation of MnO2 coated LF and LIG electrode as a function of voltage scan rate
To evaluate the performance of supercapacitors under realistic operating conditions, we conducted measurements on the full cell configuration using LF as both the anode and cathode. Figure 8a illustrates the rectangular CV curve characteristics of the LF-based full-cell supercapacitor across a range of voltage scan conditions, ranging from 50 mV/s to 400 mV/s. The galvanic charge-discharge curves depicted in Fig. 8b exhibit a quasi-symmetric triangular shape, indicating an ideal capacitive behavior, with minimal IR drop. These findings further confirm the excellent capacitive characteristics of LF-based supercapacitors. As shown in Fig. 8c, the capacitance reduction with increasing voltage scan rate in the range of 25–400 mV/s is observed, and the maximum capacitance of 275 µF is achieved at a scan rate of 25 mV/s. Furthermore, to examine the durability, which is a critical factor in the performance of supercapacitors, we measured the coulomb efficiency over 3000 cycles. The results showed that the coulomb efficiency remained at 99% or above between 300 and 3000 cycles, demonstrating excellent stability of the LF-based supercapacitor (Fig. 8d).
a CV curves of full cell LF (FCLF) in response to different scan rates. b Galvanostatic charge-discharge (GCD) curves of FCLF at different input current. c Capacitance variation of FCLF as a function of voltage scan rate. d Coulombic efficiency of FCLF versus number of cycles. The inset figure illustrates the experimental setup utilized for performance measurement of the full cell supercapacitors
3 Conclusions
In conclusion, this study has demonstrated that pyrolytic jetting process and subsequent LF based supercapacitors have great potential as a viable solution for energy storage needs, particularly when the available resources, e.g., laser fluence and raw material, are limited. The resultant LF, which is exfoliated from the mother substrate and freestanding, enables expanded surface area that is in direct contact to the electrolyte to maximize the performance of energy devices including supercapacitor presented in this study. By utilizing the free-standing characteristic of the LF, we achieved omnidirectional plating of an additional functional layer to further improve the available capacitance. However, it is worth noting that LF’s enlarged volume and independence from the substrate imply that its mechanical strength may be relatively lower compared to LIG. Therefore, it becomes imperative to undertake future studies to enhance the mechanical properties of LF. We expect that these findings can provide new insight into developing efficient and cost-effective [44] energy storage devices from the perspective of precision material processing.
Data availability
The generated and analyzed datasets during this study are available from the corresponding authors upon reasonable request.
References
Elio, J., et al. (2021). A review of energy storage technologies for demand-side management in industrial facilities. Journal of Cleaner Production, 307, 127322.
Koohi-Fayegh, S., & Rosen, M. A. (2020). A review of energy storage types, applications and recent developments. Journal of Energy Storage, 27, 101047.
Matos, C. R., Carneiro, J. F., & Silva, P. P. (2019). Overview of large-scale Underground Energy Storage Technologies for Integration of renewable energies and Criteria for Reservoir Identification. Journal of Energy Storage, 21, 241–258.
Fleischmann, S., et al. (2020). Pseudocapacitance: From Fundamental understanding to High Power Energy Storage materials. Chemical Reviews, 120(14), 6738–6782.
Serra, R., et al. (2019). From conventional to renewable natural gas: Can we expect GHG savings in the near term? Biomass and Bioenergy, 131, 105396.
Wang, F., et al. (2021). Technologies and perspectives for achieving carbon neutrality. The Innovation, 2(4), 100180.
Cherp, A., et al. (2021). National growth dynamics of wind and solar power compared to the growth required for global climate targets. Nature Energy, 6(7), 742–754.
Hasan, M. K., et al. (2021). Review of electric vehicle energy storage and management system: Standards, issues, and challenges. Journal of Energy Storage, 41, 102940.
Hu, J., et al. (2020). Intelligent energy management strategy of hybrid energy storage system for electric vehicle based on driving pattern recognition. Energy, 198, 117298.
Liserre, M., Sauter, T., & Hung, J. Y. (2010). Future Energy Systems: Integrating renewable energy sources into the Smart Power Grid through Industrial Electronics. IEEE Industrial Electronics Magazine, 4(1), 18–37.
Cañas, A. I., & Camarero, S. (2010). Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnology Advances, 28(6), 694–705.
Pomerantseva, E., et al. (2019). Energy storage: The future enabled by nanomaterials. Science, 366(6468), eaan8285.
Miller, J. R., & Simon, P. (2008). Electrochemical Capacitors for Energy Management. Science, 321(5889), 651–652.
Chmiola, J., et al. (2006). Anomalous increase in Carbon Capacitance at Pore Sizes Less Than 1 nanometer. Science, 313(5794), 1760–1763.
Raymundo-Piñero, E., et al. (2006). Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon, 44(12), 2498–2507.
Zhu, Y., et al. (2011). Carbon-Based supercapacitors produced by activation of Graphene. Science, 332(6037), 1537–1541.
Merlet, C., et al. (2013). Highly confined ions store charge more efficiently in supercapacitors. Nature Communications, 4(1), 2701.
Liu, C., et al. (2010). Graphene-Based supercapacitor with an Ultrahigh Energy Density. Nano Letters, 10(12), 4863–4868.
Olabi, A. G., et al. (2021). Application of graphene in energy storage device – A review. Renewable and Sustainable Energy Reviews, 135, 110026.
Wang, Y., et al. (2009). Supercapacitor Devices based on Graphene materials. The Journal of Physical Chemistry C, 113(30), 13103–13107.
In, J. B., et al. (2015). Facile fabrication of flexible all solid-state micro-supercapacitor by direct laser writing of porous carbon in polyimide. Carbon, 83, 144–151.
Li, L., et al. (2016). High-performance Pseudocapacitive Microsupercapacitors from Laser-Induced Graphene. Advanced Materials, 28(5), 838–845.
Barbhuiya, N. H., Kumar, A., & Singh, S. P. (2021). A Journey of Laser-Induced Graphene in Water Treatment. Transactions of the Indian National Academy of Engineering, 6(2), 159–171.
Jung, Y., et al. (2022). Smart paper electronics by laser-induced graphene for biodegradable real-time food spoilage monitoring. Applied Materials Today, 29, 101589.
Le, T. S. D., et al. (2022). Recent advances in Laser-Induced Graphene: Mechanism, fabrication, Properties, and applications in Flexible Electronics. Advanced Functional Materials, 32(48), 2205158.
Cardoso, A. R. (2019). Molecularly-imprinted chloramphenicol sensor with laser-induced graphene electrodes. Biosensors and Bioelectronics, 124–125: p. 167–175.
Cheng, C., et al. (2016). Bisphenol A Sensors on Polyimide fabricated by Laser Direct writing for Onsite River Water Monitoring at Attomolar Concentration. ACS Applied Materials & Interfaces, 8(28), 17784–17792.
Lamberti, A., et al. (2016). A highly stretchable Supercapacitor using Laser-Induced Graphene Electrodes onto Elastomeric substrate. Advanced Energy Materials, 6(10), 1600050.
Lim, J., et al. (2022). Monolithic digital patterning of polyimide by laser-induced pyrolytic jetting. Chemical Engineering Journal, 428, 131050.
Varma, J. (2018). Fiber-type solar cells, nanogenerators, batteries, and Supercapacitors for Wearable Applications. Advanced Science, 5(9), 1800340.
Yin, J., et al. (2020). Synthesis strategies of porous Carbon for Supercapacitor Applications. Small Methods, 4(3), 1900853.
Lin, J., et al. (2014). Laser-induced porous graphene films from commercial polymers. Nature Communications, 5(1), 5714.
Bäuerle, D. (2013). Laser processing and chemistry. Springer Science & Business Media.
Shin, J., et al. (2021). Monolithic digital patterning of polydimethylsiloxane with successive laser pyrolysis. Nature Materials, 20(1), 100–107.
Lee, J., et al. (2022). Facile Fabrication of High-Performance Hybrid Supercapacitor by One-Step, self‐grown copper Nanopillar Forest anchored with Fe3O4 anode. International Journal of Precision Engineering and Manufacturing-Green Technology, 9(1), 213–223.
Vivaldi, F. M., et al. (2021). Three-Dimensional (3D) Laser-Induced Graphene: Structure, Properties, and application to Chemical sensing. ACS Applied Materials & Interfaces, 13(26), 30245–30260.
Wang, H., et al. (2022). A soft and stretchable electronics using laser-induced graphene on polyimide/PDMS composite substrate. npj Flexible Electronics, 6(1), 26.
Childres, I. (2013). Raman Spectroscopy of Graphene and Related Materials New Developments in Photon and Materials Research, : p. 403–418.
Hwang, Y. T., & Kim, H. S. (2022). The Ultrafast and eco-friendly reduction of Graphene Oxide using a UV–IR assisted intense pulsed light and its application as Supercapacitor. International Journal of Precision Engineering and Manufacturing-Green Technology, 9(1), 201–211.
Aliofkhazraei, M. (2016). Graphene Science Handbook: Fabrication Methods.
Lau, D., et al. (2009). Microstructural investigation supporting an abrupt stress induced transformation in amorphous carbon films. Journal of Applied Physics, 105, 084302–084302.
Yoo, H. M., Heo, G. Y., & Park, S. J. (2011). Effect of crystallinity on the electrochemical properties of carbon black electrodes. Carbon Letters, 12(4), 252–255.
Geim, A. K. (2009). Graphene: Status and prospects. Science, 324(5934), 1530–1534.
Kim, J., et al. (2023). Waterproof and wear-resistant surface treatment on printed parts of Polyamide 12 (PA12) by selective laser sintering using a large Pulsed Electron Beam. International Journal of Precision Engineering and Manufacturing-Green Technology, 10(1), 71–83.
Acknowledgements
This research is supported by a 2 year Research Grant of Pusan National University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, D., Lee, H., Hwang, E. et al. Pyrolytic Jetting of Highly Porous Laser-Induced Graphene Fiber for Cost-Effective Supercapacitor. Int. J. of Precis. Eng. and Manuf.-Green Tech. 11, 439–447 (2024). https://doi.org/10.1007/s40684-023-00566-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40684-023-00566-9