Abstract
Purpose of Review
The purpose of the study is to summarize the potential interactions between obstructive sleep apnea (OSA), atrial fibrillation (AF), and connexins.
Recent Findings
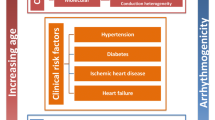
OSA is highly prevalent in patients with cardiovascular disease, and is associated with increased risk for end-organ substantial morbidities linked to autonomic nervous system imbalance and increased oxidative stress and inflammation, ultimately leading to reduced life expectancy. Epidemiological studies indicate that OSA is associated with increased incidence and progression of coronary heart disease, heart failure, stroke, as well as arrhythmias, particularly AF. Conversely, AF is very common among subjects referred for suspected OSA, and the prevalence of AF increases with OSA severity. The interrelationships between AF and OSA along with the well-known epidemiological links between these two conditions and obesity may reflect shared pathophysiological pathways, which may depend on the intercellular diffusion of signaling molecules into either the extracellular space or require cell-to-cell contact. Connexin signaling is accomplished via direct exchanges of cytosolic molecules between adjacent cells at gap membrane junctions for cell-to-cell coupling. The role of connexins in AF is now quite well established, but the impact of OSA on cardiac connexins has only recently begun to be investigated. Understanding the biology and regulatory mechanisms of connexins in OSA at the transcriptional, translational, and post-translational levels will undoubtedly require major efforts to decipher the breadth and complexity of connexin functions in OSA-induced AF.
Summary
The risk of end-organ morbidities has initiated the search for circulating mechanistic biomarker signatures and the implementation of biomarker-based algorithms for precision-based diagnosis and risk assessment. Here, we summarize recent findings in OSA as they relate to AF risk, and also review potential mechanisms linking OSA, AF, and connexins.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Hnin K, Mukherjee S, Antic NA, Catcheside P, Chai-Coetzer CL, McEvoy D, et al. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med Rev. 2018;41:78–86. This is a recent review provding an overveiw about the impact of ethnicity on the severity of OSA pateints.
Peppard PE, Hagen EW. The last 25 years of obstructive sleep apnea epidemiology-and the next 25? Am J Respir Crit Care Med. 2018;197:310–2.
Levy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015.
Leger D, Bayon V, Laaban JP, Philip P. Impact of sleep apnea on economics. Sleep Med Rev. 2012;16:455–62.
Jehan S, Zizi F, Pandi-Perumal SR, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. 2017;1(4):1–14
Geovanini GR, Wang R, Weng J, et al. Association between obstructive sleep apnea and cardiovascular risk factors: variation by age, sex, and race. The multi-ethnic study of atherosclerosis. Ann Am Thorac Soc. 2018;15:970–7.
Levy P, Ryan S, Oldenburg O, Parati G. Sleep apnoea and the heart. Eur Respir Rev. 2013;22:333–52.
Ando SI. Influence of hypoxia induced by sleep disordered breathing in case of hypertension and atrial fibrillation. J Cardiol. 2018;72:10–8.
Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36.
Parati G, Lombardi C, Hedner J, et al. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J. 2013;41:523–38.
Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1:61–72.
Javaheri S, Campos-Rodriguez F. Outcomes of positive airway pressure for sleep apnea. JAMA. 2017;318:2042–3.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14.
Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2014;307:L129–40.
Khalyfa A, Qiao Z, Gileles-Hillel A, et al. Activation of the integrated stress response and metabolic dysfunction in a murine model of sleep apnea. Am J Respir Cell Mol Biol. 2017;57:477–86.
Khalyfa A, Youssefnia N, Foster GE, et al. Plasma Exosomes and improvements in endothelial function by angiotensin 2 type 1 receptor or cyclooxygenase 2 blockade following intermittent hypoxia. Front Neurol. 2017;8:709.
Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. 2015;16:25–34.
Schulz R, Murzabekova G, Egemnazarov B, et al. Arterial hypertension in a murine model of sleep apnea: role of NADPH oxidase 2. J Hypertens. 2014;32:300–5.
Drager LF, Yao Q, Hernandez KL, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–8.
Gileles-Hillel A, Almendros I, Khalyfa A, Nigdelioglu R, Qiao Z, Hamanaka RB, et al. Prolonged exposures to intermittent hypoxia promote visceral white adipose tissue inflammation in a murine model of severe sleep apnea: effect of normoxic recovery. Sleep. 2017;40.1–10.
Gharib SA, Khalyfa A, Abdelkarim A, et al. Intermittent hypoxia activates temporally coordinated transcriptional programs in visceral adipose tissue. J Mol Med. 2012;90:435–45.
Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol. 2009;106:1538–44.
Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension. 2010;56:369–77.
Beaudin AE, Pun M, Yang C, et al. Cyclooxygenases 1 and 2 differentially regulate blood pressure and cerebrovascular responses to acute and chronic intermittent hypoxia: implications for sleep apnea. J Am Heart Assoc. 2014;3:e000875.
Champod AS, Eskes GA, Foster GE, et al. Effects of acute intermittent hypoxia on working memory in young healthy adults. Am J Respir Crit Care Med. 2013;187:1148–50.
Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402–12.
Tuomilehto H, Seppa J, Uusitupa M. Obesity and obstructive sleep apnea—clinical significance of weight loss. Sleep Med Rev. 2013;17:321–9.
Hu Y, Bhupathiraju SN, de Koning L, Hu FB. Duration of obesity and overweight and risk of type 2 diabetes among US women. Obesity. 2014;22:2267–73.
Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–89.
Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–74.
Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80.
Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8.
Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–12.
Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:653789.
Thurnheer R, Wraith PK, Douglas NJ. Influence of age and gender on upper airway resistance in NREM and REM sleep. J Appl Physiol. 2001;90:981–8.
Baum DM, Morales Rodriguez B, Attali V, Cauhape M, Arnulf I, Cardot P, et al. New Zealand obese mice as translational model of obesity-related obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2018. https://doi.org/10.1164/rccm.201801-0162LE.
Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–71.
Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5.
Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–7.
Togeiro SM, Carneiro G, Ribeiro Filho FF, et al. Consequences of obstructive sleep apnea on metabolic profile: a population-based survey. Obesity. 2013;21:847–51.
Mongraw-Chaffin M, Foster MC, Anderson CAM, et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2018;71:1857–65.
Bober SL, Ciriello J, Jones DL. Atrial arrhythmias and autonomic dysfunction in rats exposed to chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2018;314:H1160–H8.
Estrada JA, Barlow MA, Yoshishige D, et al. Delta-opioid receptors: pivotal role in intermittent hypoxia-augmentation of cardiac parasympathetic control and plasticity. Auton Neurosci. 2016;198:38–49.
Ferreira CB, Schoorlemmer GH, Rossi MV, et al. Brainstem areas activated by intermittent apnea in awake unrestrained rats. Neuroscience. 2015;297:262–71.
Dergacheva O, Dyavanapalli J, Pinol RA, Mendelowitz D. Chronic intermittent hypoxia and hypercapnia inhibit the hypothalamic paraventricular nucleus neurotransmission to parasympathetic cardiac neurons in the brain stem. Hypertension. 2014;64:597–603.
Dyavanapalli J, Jameson H, Dergacheva O, Jain V, Alhusayyen M, Mendelowitz D. Chronic intermittent hypoxia-hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons. J Physiol. 2014;592:2799–811.
Chalacheva P, Thum J, Yokoe T, O’Donnell CP, Khoo MC. Development of autonomic dysfunction with intermittent hypoxia in a lean murine model. Respir Physiol Neurobiol. 2013;188:143–51.
Lu Z, Nie L, He B, et al. Increase in vulnerability of atrial fibrillation in an acute intermittent hypoxia model: importance of autonomic imbalance. Auton Neurosci. 2013;177:148–53.
Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–7.
Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620–6.
Todd K, McIntyre WF, Baranchuk A. Obstructive sleep apnea and atrial fibrillation. Nature Sci Sleep. 2010;2:39–45.
Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84.
Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25.
Memtsoudis SG, Stundner O, Rasul R, et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg. 2014;118:407–18.
Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6.
Mehra R, Wang L, Andrews N, et al. Dissociation of objective and subjective daytime sleepiness and biomarkers of systemic inflammation in sleep-disordered breathing and systolic heart failure. J Clin Sleep Med. 2017;13:1411–22.
Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8.
Costa LE, Uchoa CH, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart. 2015;101:1288–92.
Zhao LP, Kofidis T, Lim TW, et al. Sleep apnea is associated with new-onset atrial fibrillation after coronary artery bypass grafting. J Crit Care. 2015;30:1418 e1–5.
Ghias M, Scherlag BJ, Lu Z, et al. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009;54:2075–83.
Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–7.
Sapina E, Torres G, Barbe F, Sanchez-de-la-Torre M. The use of precision medicine to manage obstructive sleep apnea treatment in patients with resistant hypertension: current evidence and future directions. Curr Hypertens Rep. 2018;20:60.
Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15.
Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8.
•• Sanchez-de-la-Torre M, Khalyfa A, Sanchez-de-la-Torre A, et al. Precision medicine in patients with resistant hypertension and obstructive sleep apnea: blood pressure response to continuous positive airway pressure treatment. J Am Coll Cardiol. 2015;66:1023–32. This study provides an evidence about how miRNA signature can be used in a precision medicine in OSA pateints with with hybertension.
Lim DC, Sutherland K, Cistulli PA, Pack AI. P4 medicine approach to obstructive sleep apnoea. Respirology. 2017;22:849–60.
Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–9.
Linssen GC, Rienstra M, Jaarsma T, et al. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2011;13:1111–20.
Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47.
Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–27.
Karayiannides S, Lundman P, Friberg L, Norhammar A. High overall cardiovascular risk and mortality in patients with atrial fibrillation and diabetes: a nationwide report. Diab Vasc Dis Res. 2018;15:31–8.
Pellman J, Sheikh F. Atrial fibrillation: mechanisms, therapeutics, and future directions. Compr Physiol. 2015;5:649–65.
Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26.
Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–16.
Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325.
Wasmer K, Eckardt L, Breithardt G. Predisposing factors for atrial fibrillation in the elderly. J Geriatr Cardiol. 2017;14:179–84.
Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–5.
Ko YJ, Kim S, Park K, et al. Impact of the health insurance coverage policy on Oral anticoagulant prescription among patients with atrial fibrillation in Korea from 2014 to 2016. J Korean Med Sci. 2018;33:e163.
McManus DD, Xanthakis V, Sullivan LM, et al. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation. 2010;121:667–74.
Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7.
Camm AJ, Savelieva I, Potpara T, Hindriks G, Pison L, Blomstrom-Lundqvist C. The changing circumstance of atrial fibrillation—progress towards precision medicine. J Intern Med. 2016;279:412–27.
• Marulanda-Londono E, Chaturvedi S. The interplay between obstructive sleep apnea and atrial fibrillation. Front Neurol. 2017;8:668. This review provides details about the interplay between OSA and artirial fibrillation.
Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;155:310–5.
Zhang L, Hou Y, Po SS. Obstructive sleep apnoea and atrial fibrillation. Arrhythm Electrophysiol Rev. 2015;4:14–8.
Maan A, Mansour M, Anter E, et al. Obstructive sleep apnea and atrial fibrillation: pathophysiology and implications for treatment. Crit Pathw Cardiol. 2015;14:81–5.
Goyal SK, Sharma A. Atrial fibrillation in obstructive sleep apnea. World J Cardiol. 2013;5:157–63.
Latina JM, Estes NA 3rd, Garlitski AC. The relationship between obstructive sleep apnea and atrial fibrillation: a complex interplay. Pulm Med. 2013;2013:621736.
Fung JW, Li TS, Choy DK, et al. Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest. 2002;121:422–9.
Di Pasquale G, Mathieu G, Maggioni AP, et al. Current presentation and management of 7148 patients with atrial fibrillation in cardiology and internal medicine hospital centers: the ATA AF study. Int J Cardiol. 2013;167:2895–903.
Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20.
Wyse DG, Van Gelder IC, Ellinor PT, et al. Lone atrial fibrillation: does it exist? J Am Coll Cardiol. 2014;63:1715–23.
Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–99.
Calvo D, Filgueiras-Rama D, Jalife J. Mechanisms and drug development in atrial fibrillation. Pharmacol Rev. 2018;70:505–25.
Zacharia E, Papageorgiou N, Ioannou A, et al. Inflammatory biomarkers in atrial fibrillation. Curr Med Chem. 2017. https://doi.org/10.2174/0929867324666170727103357.
Wolk R, Kara T, Somers VK. Sleep-disordered breathing and cardiovascular disease. Circulation. 2003;108:9–12.
Digby GC, Baranchuk A. Sleep apnea and atrial fibrillation; 2012 update. Curr Cardiol Rev. 2012;8:265–72.
Rossi VA, Stradling JR, Kohler M. Effects of obstructive sleep apnoea on heart rhythm. Eur Respir J. 2013;41:1439–51.
Gutierrez A, Van Wagoner DR. Oxidant and inflammatory mechanisms and targeted therapy in atrial fibrillation: an update. J Cardiovasc Pharmacol. 2015;66:523–9.
Youn JY, Zhang J, Zhang Y, et al. Oxidative stress in atrial fibrillation: an emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–9.
Linz D, Schotten U, Neuberger HR, Bohm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8:1436–43.
Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54:1797–804.
Lavergne F, Morin L, Armitstead J, Benjafield A, Richards G, Woehrle H. Atrial fibrillation and sleep-disordered breathing. J Thorac Dis. 2015;7:E575–84.
Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662–9.
Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53.
Bitter T, Nolker G, Vogt J, Prinz C, Horstkotte D, Oldenburg O. Predictors of recurrence in patients undergoing cryoballoon ablation for treatment of atrial fibrillation: the independent role of sleep-disordered breathing. J Cardiovasc Electrophysiol. 2012;23:18–25.
Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin. 2009;27:13–24 vii.
Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm. 2010;7:1263–70.
Guasch E, Benito B, Qi X, et al. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol. 2013;62:68–77.
Iwasaki YK, Kato T, Xiong F, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol. 2014;64:2013–23.
Pepin JL, Levy P. Pathophysiology of cardiovascular risk in sleep apnea syndrome (SAS). Rev Neurol (Paris). 2002;158:785–97.
Lira AB, de Sousa Rodrigues CF. Evaluation of oxidative stress markers in obstructive sleep apnea syndrome and additional antioxidant therapy: a review article. Sleep Breath. 2016;20:1155–60.
Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2:e000421.
Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647–54 e2.
Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331–7.
Farre N, Otero J, Falcones B, et al. Intermittent hypoxia mimicking sleep apnea increases passive stiffness of myocardial extracellular matrix. A multiscale study. Front Physiol. 2018;9:1143.
Daccarett M, McGann CJ, Akoum NW, MacLeod RS, Marrouche NF. MRI of the left atrium: predicting clinical outcomes in patients with atrial fibrillation. Expert Rev Cardiovasc Ther. 2011;9:105–11.
Latini R, Masson S, Pirelli S, et al. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med. 2011;269:160–71.
Riley G, Syeda F, Kirchhof P, Fabritz L. An introduction to murine models of atrial fibrillation. Front Physiol. 2012;3:296.
• Aasen T, Johnstone S, Vidal-Brime L, Lynn KS, Koval M. Connexins: synthesis, post-translational modifications, and trafficking in health and disease. Int J Mol Sci. 2018;19:1–36. This is a recent review about connexins in health and diseases conditions.
Leybaert L, Lampe PD, Dhein S, et al. Connexins in cardiovascular and neurovascular health and disease: pharmacological implications. Pharmacol Rev. 2017;69:396–478.
Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–32.
Liu H, Radisky DC, Bissell MJ. Proliferation and polarity in breast cancer: untying the Gordian knot. Cell Cycle. 2005;4:646–9.
Gros DB, Jongsma HJ. Connexins in mammalian heart function. BioEssays. 1996;18:719–30.
Desplantez T. Cardiac Cx43, Cx40 and Cx45 co-assembling: involvement of connexins epitopes in formation of hemichannels and gap junction channels. BMC Cell Biol. 2017;18:3.
Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid Redox Signal. 2009;11:267–82.
Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiol Rev. 2011;91:1393–445.
Ongstad E, Kohl P. Fibroblast-myocyte coupling in the heart: potential relevance for therapeutic interventions. J Mol Cell Cardiol. 2016;91:238–46.
Beauchamp P, Yamada KA, Baertschi AJ, et al. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ Res. 2006;99:1216–24.
Severs NJ, Dupont E, Coppen SR, et al. Remodelling of gap junctions and connexin expression in heart disease. Biochim Biophys Acta. 2004;1662:138–48.
Avanzo JL, Mesnil M, Hernandez-Blazquez FJ, et al. Altered expression of connexins in urethane-induced mouse lung adenomas. Life Sci. 2006;79:2202–8.
Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–43.
Firouzi M, Bierhuizen MF, Kok B, et al. The human Cx40 promoter polymorphism -44G-->A differentially affects transcriptional regulation by Sp1 and GATA4. Biochim Biophys Acta. 2006;1759:491–6.
Firouzi M, Kok B, Spiering W, et al. Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens. 2006;24:325–30.
Firouzi M, Ramanna H, Kok B, et al. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ Res. 2004;95:e29–33.
Richard G. Connexin disorders of the skin. Clin Dermatol. 2005;23:23–32.
Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell. 1993;4:7–20.
Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–43.
van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112:479–86.
de Wit C, Roos F, Bolz SS, et al. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–55.
Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–63.
Reaume AG, de Sousa PA, Kulkarni S, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–4.
Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochim Biophys Acta. 2005;1719:6–23.
D’Hondt C, Iyyathurai J, Vinken M, et al. Regulation of connexin- and pannexin-based channels by post-translational modifications. Biol Cell. 2013;105:373–98.
Oyamada M, Takebe K, Oyamada Y. Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochim Biophys Acta. 2013;1828:118–33.
Vinken M, De Rop E, Decrock E, et al. Epigenetic regulation of gap junctional intercellular communication: more than a way to keep cells quiet? Biochim Biophys Acta. 2009;1795:53–61.
Piechocki MP, Toti RM, Fernstrom MJ, Burk RD, Ruch RJ. Liver cell-specific transcriptional regulation of connexin32. Biochim Biophys Acta. 2000;1491:107–22.
Johnstone S, Isakson B, Locke D. Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol. 2009;278:69–118.
Straub AC, Johnstone SR, Heberlein KR, et al. Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J Vasc Res. 2010;47:277–86.
Nagibin V, Egan Benova T, Viczenczova C, et al. Ageing related down-regulation of myocardial connexin-43 and up-regulation of MMP-2 may predict propensity to atrial fibrillation in experimental animals. Physiol Res. 2016;65(Suppl 1):S91–S100.
Tribulova N, Egan Benova T, Szeiffova Bacova B, Viczenczova C, Barancik M. New aspects of pathogenesis of atrial fibrillation: remodelling of intercalated discs. J Physiol Pharmacol. 2015;66:625–34.
Santa Cruz A, Mese G, Valiuniene L, Brink PR, White TW, Valiunas V. Altered conductance and permeability of Cx40 mutations associated with atrial fibrillation. J Gen Physiol. 2015;146:387–98.
Bruegmann T, Beiert T, Vogt CC, Schrickel JW, Sasse P. Optogenetic termination of atrial fibrillation in mice. Cardiovasc Res. 2018;114:713–23.
Zhang F, Bian Y, Huang L, Fan W. Association between connexin 40 and potassium voltage-gated channel subfamily A member 5 expression in the atrial myocytes of patients with atrial fibrillation. Exp Ther Med. 2017;14:5170–6.
Shu C, Huang W, Zeng Z, et al. Connexin 43 is involved in the sympathetic atrial fibrillation in canine and canine atrial myocytes. Anatol J Cardiol. 2017;18:3–9.
Kanthan A, Fahmy P, Rao R, et al. Human connexin40 mutations slow conduction and increase propensity for atrial fibrillation. Heart Lung Circ. 2018;27:114–21.
Gemel J, Su Z, Gileles-Hillel A, Khalyfa A, Gozal D, Beyer EC. Intermittent hypoxia causes NOX2-dependent remodeling of atrial connexins. BMC Cell Biol. 2017;18:7.
Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, et al. Corrigendum: gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep. 2015;5:14888.
Ribeiro-Rodrigues TM, Martins-Marques T, Morel S, Kwak BR, Girao H. Role of connexin 43 in different forms of intercellular communication—gap junctions, extracellular vesicles and tunnelling nanotubes. J Cell Sci. 2017;130:3619–30.
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39.
Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–8.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6.
Michael A, Bajracharya SD, Yuen PS, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–8.
Khalyfa A, Khalyfa AA, Akbarpour M, Connes P, Romana M, Laping-Carr G, et al. Extracellular microvesicle microRNAs in children with sickle cell anemia with divergent clinical phenotypes. Br Haematol. 2016; in press.
Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, et al. Circulating plasma extracellular microvesicle miRNA cargo and endothelial dysfunction in OSA children. Am J Respir Crit Care Med. 2016; In press.
Khalyfa A, Almendros I, Gileles-Hillel A, et al. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget. 2016;7:54676–90.
Almendros I, Khalyfa A, Trzepizur W, et al. Tumor cell malignant properties are enhanced by circulating exosomes in sleep apnea. Chest. 2016;150:1030–41.
Khalyfa A, Kheirandish-Gozal L, Gozal D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir Physiol Neurobiol. 2017;256:143–156.
•• Khalyfa A, Gozal D, Masa JF, Marin JM, Qiao Z, Corral J, et al. Sleep-disordered breathing, circulating exosomes, and insulin sensitivity in adipocytes. Int J Obes. 2018;42:1127–39. This is the most recent study shows using in vitro adipocyte-based functional reporter assays, alterations in plasma exosomal cargo occur in SDB, and appear to contribute to adipocyte metabolic dysfunction.
Jesel L, Abbas M, Toti F, Cohen A, Arentz T, Morel O. Microparticles in atrial fibrillation: a link between cell activation or apoptosis, tissue remodelling and thrombogenicity. Int J Cardiol. 2013;168:660–9.
Sundararajan V, Sarkar FH, Ramasamy TS. The versatile role of exosomes in cancer progression: diagnostic and therapeutic implications. Cell Oncol (Dordr). 2018;41:223–52.
Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78.
Urbanelli L, Buratta S, Sagini K, Ferrara G, Lanni M, Emiliani C. Exosome-based strategies for diagnosis and therapy. Recent Pat CNS Drug Discov. 2015;10:10–27.
Funding
This work was supported in part by National Institutes of Health grant HL130984 and the Marie M. and Harry L. Smith Endowed Chair in Child Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Abdelnaby Khalyfa and David Gozal declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sleep Related Breathing Disorders
Rights and permissions
About this article
Cite this article
Khalyfa, A., Gozal, D. Connexins and Atrial Fibrillation in Obstructive Sleep Apnea. Curr Sleep Medicine Rep 4, 300–311 (2018). https://doi.org/10.1007/s40675-018-0130-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-018-0130-7