Abstract
Purpose of Review
In the recent decade, new animal models which phenocopy a broad range of systemic sclerosis (SSc)-like features have been generated. This review article outlines the strengths and limitations of 4 animal models, which were established or further characterized in the recent few years.
Recent Findings
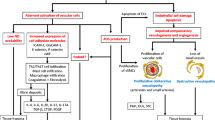
Inducible SSc animal models, such as hypochlorous acid-treated mice and type V collagen-immunized mice and rabbits, recapitulate multiple organ involvement of SSc, including diffuse skin fibrosis, interstitial lung disease, pulmonary arterial hypertension, and/or kidney involvement. Further characterization of vascular aspects in Psgl1−/− mice shed new light on the endothelium-leukocyte interaction in immune tolerance underlying SSc-like vasculopathy. In vitro studies with dermal microvascular endothelial cells, bone marrow-derived endothelial progenitor cells, and bone marrow-derived mesenchymal stem cells, a precursor of pericytes, derived from Klf5+/−;Fli1+/− mice dissect the aberrant roles of angiogenesis, vasculogenesis, and anastomosis in SSc-like vasculopathy.
Summary
SSc animal models which reproduce a broad range of organ involvement support our understanding of an SSc-specific pathological cascade, but no current animal models mimic the whole range of SSc pathological features. However, a proper combination of complimentary models can help us better understand the molecular pathogenesis of SSc and promote the development of new therapies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–99.
Asano Y. Systemic sclerosis. J Dermatol. 2018;45(2):128–38.
Asano Y. The pathogenesis of systemic sclerosis: an understanding based on a common pathologic cascade across multiple organs and additional organ-specific pathologies. J Clin Med. 2020;9(9).
Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297(8):333–44.
Yoshizaki A, Yanaba K, Ogawa A, Asano Y, Kadono T, Sato S. Immunization with DNA topoisomerase I and Freund’s complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheum. 2011;63(11):3575–85.
•• Teodoro WR, de Jesus Queiroz ZA, Dos Santos LA, Catanozi S, Dos Santos Filho A, Bueno C, et al. Proposition of a novel animal model of systemic sclerosis induced by type V collagen in C57BL/6 mice that reproduces fibrosis, vasculopathy and autoimmunity. Arthritis Res Ther. 2019;21(1):278. This article demonstrates that the three cardinal pathological features of systemic sclerosis, including autoimmunity, vasculopathy and extensive tissue fibrosis, can be induced by immunization of type V collagen in mice, as is the case with rabbits.
Teodoro WR, Velosa AP, Witzel SS, Garippo AL, Farhat C, Parra ER, et al. Architectural remodelling in lungs of rabbits induced by type V collagen immunization: a preliminary morphologic model to study diffuse connective tissue diseases. Pathol Res Pract. 2004;200(10):681–91.
• Meng M, Tan J, Chen W, Du Q, Xie B, Wang N, et al. The fibrosis and immunological features of hypochlorous acid induced mouse model of systemic sclerosis. Front Immunol. 2019;10:1861. This article demonstrates that a hypochlorous acid induced murine model displays systemic immune cell infiltration, pro-inflammatory mediator release, vasculopathy and fibrosis, which mimics those of systemic sclerosis.
Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82(3):493–512.
Christner PJ, Peters J, Hawkins D, Siracusa LD, Jiménez SA. The tight skin 2 mouse. An animal model of scleroderma displaying cutaneous fibrosis and mononuclear cell infiltration. Arthritis Rheum. 1995;38(12):1791–8.
Maurer B, Distler JH, Distler O. The Fra-2 transgenic mouse model of systemic sclerosis. Vascul Pharmacol. 2013;58(3):194–201.
Manetti M, Rosa I, Milia AF, Guiducci S, Carmeliet P, Ibba-Manneschi L, et al. Inactivation of urokinase-type plasminogen activator receptor (uPAR) gene induces dermal and pulmonary fibrosis and peripheral microvasculopathy in mice: a new model of experimental scleroderma? Ann Rheum Dis. 2014;73(9):1700–9.
Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, et al. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007;56(1):334–44.
Denton CP, Zheng B, Evans LA, Shi-wen X, Ong VH, Fisher I, et al. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor beta (TGFbeta) receptor leads to paradoxical activation of TGFbeta signaling pathways with fibrosis in transgenic mice. J Biol Chem. 2003;278(27):25109–19.
Sonnylal S, Shi-Wen X, Leoni P, Naff K, Van Pelt CS, Nakamura H, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62(5):1523–32.
Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279(34):35503–9.
Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, et al. β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis. 2012;71(5):761–7.
Asano Y, Stawski L, Hant F, Highland K, Silver R, Szalai G, et al. Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am J Pathol. 2010;176(4):1983–98.
Takahashi T, Asano Y, Sugawara K, Yamashita T, Nakamura K, Saigusa R, et al. Epithelial Fli1 deficiency drives systemic autoimmunity and fibrosis: Possible roles in scleroderma. J Exp Med. 2017;214(4):1129–51.
Noda S, Asano Y, Nishimura S, Taniguchi T, Fujiu K, Manabe I, et al. Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat Commun. 2014;5:5797.
Sgonc R, Gruschwitz MS, Dietrich H, Recheis H, Gershwin ME, Wick G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996;98(3):785–92.
Maurer B, Distler A, Suliman YA, Gay RE, Michel BA, Gay S, et al. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis. 2014;73(10):1880–7.
Parapuram SK, Shi-wen X, Elliott C, Welch ID, Jones H, Baron M, et al. Loss of PTEN expression by dermal fibroblasts causes skin fibrosis. J Invest Dermatol. 2011;131(10):1996–2003.
Parapuram SK, Thompson K, Tsang M, Hutchenreuther J, Bekking C, Liu S, et al. Loss of PTEN expression by mouse fibroblasts results in lung fibrosis through a CCN2-dependent mechanism. Matrix Biol. 2015;43:35–41.
Pérez-Frías A, González-Tajuelo R, Núñez-Andrade N, Tejedor R, García-Blanco MJ, Vicente-Rabaneda E, et al. Development of an autoimmune syndrome affecting the skin and internal organs in P-selectin glycoprotein ligand 1 leukocyte receptor-deficient mice. Arthritis Rheumatol. 2014;66(11):3178–89.
Mak KM, Png CY, Lee DJ. Type V collagen in health, disease, and fibrosis. Anat Rec (Hoboken). 2016;299(5):613–29.
Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91(3):326–35.
Bowen JM, Sobey GJ, Burrows NP, Colombi M, Lavallee ME, Malfait F, et al. Ehlers-Danlos syndrome, classical type. Am J Med Genet C Semin Med Genet. 2017;175(1):27–39.
Liu T, Zhang J. Detection of V, III and I type collagens of dermal tissues in skin lesions of patients with systemic sclerosis and its implication. J Huazhong Univ Sci Technolog Med Sci. 2008;28(5):599–603.
Parra ER, Teodoro WR, Velosa AP, de Oliveira CC, Yoshinari NH, Capelozzi VL. Interstitial and vascular type V collagen morphologic disorganization in usual interstitial pneumonia. J Histochem Cytochem. 2006;54(12):1315–25.
Martin P, Teodoro WR, Velosa AP, de Morais J, Carrasco S, Christmann RB, et al. Abnormal collagen V deposition in dermis correlates with skin thickening and disease activity in systemic sclerosis. Autoimmun Rev. 2012;11(11):827–35.
Parra ER, Teodoro WR, de Morais J, Katayama ML, de Souza R, Yoshinari NH, et al. Increased mRNA expression of collagen V gene in pulmonary fibrosis of systemic sclerosis. Eur J Clin Invest. 2010;40(2):110–20.
Parra ER, Aguiar AC Jr, Teodoro WR, de Souza R, Yoshinari NH, Capelozzi VL. Collagen V and vascular injury promote lung architectural changes in systemic sclerosis. Clin Respir J. 2009;3(3):135–42.
• Velosa APP, Brito L, de Jesus Queiroz ZA, Carrasco S, Tomaz de Miranda J, Farhat C, et al. Identification of autoimmunity to peptides of collagen V α1 chain as newly biomarkers of early stage of systemic sclerosis. Front Immunol. 2020;11:604602. This article demonstrates that the autoantibodies against the α1(V) chain, which react with SSc-ILD lung tissues, but not normal lung tissues, are frequently detectable in patients with early systemic sclerosis, suggesting that these antibodies could be biomarkers of disease stages and potential target of immunotherapy with immunogenic peptides derived from α1(V) chain.
Callado MR, Viana VS, Vendramini MB, Leon EP, Bueno C, Velosa AP, et al. Autoantibody profile in the experimental model of scleroderma induced by type V human collagen. Immunology. 2007;122(1):38–46.
Velosa AP, Teodoro WR, dos Anjos DM, Konno R, Oliveira CC, Katayama ML, et al. Collagen V-induced nasal tolerance downregulates pulmonary collagen mRNA gene and TGF-beta expression in experimental systemic sclerosis. Respir Res. 2010;11(1):1.
Mackel AM, DeLustro F, Harper FE, LeRoy EC. Antibodies to collagen in scleroderma. Arthritis Rheum. 1982;25(5):522–31.
Riente L, Marchini B, Dolcher MP, Puccetti A, Bombardieri S, Migliorini P. Anti-collagen antibodies in systemic sclerosis and in primary Raynaud’s phenomenon. Clin Exp Immunol. 1995;102(2):354–9.
• Marangoni RG, Korman BD, Parra ER, Velosa APP, Barbeiro HV, Martins V, et al. Pathological pulmonary vascular remodeling is induced by type V collagen in a model of scleroderma. Pathol Res Pract. 2021;220:153382. This article shows the reproduction of pulmonary vascular changes similar to those of pulmonary arterial hypertension associated with systemic sclerosis in rabbits immunized with type V collagen.
Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230(1):75–96.
Domínguez-Luis M, Lamana A, Vazquez J, García-Navas R, Mollinedo F, Sánchez-Madrid F, et al. The metalloprotease ADAM8 is associated with and regulates the function of the adhesion receptor PSGL-1 through ERM proteins. Eur J Immunol. 2011;41(12):3436–42.
He X, Schoeb TR, Panoskaltsis-Mortari A, Zinn KR, Kesterson RA, Zhang J, et al. Deficiency of P-selectin or P-selectin glycoprotein ligand-1 leads to accelerated development of glomerulonephritis and increased expression of CC chemokine ligand 2 in lupus-prone mice. J Immunol. 2006;177(12):8748–56.
Bullard DC, Mobley JM, Justen JM, Sly LM, Chosay JG, Dunn CJ, et al. Acceleration and increased severity of collagen-induced arthritis in P-selectin mutant mice. J Immunol. 1999;163(5):2844–9.
Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa A, Akiyama Y, et al. Cell adhesion molecules regulate fibrotic process via Th1/Th2/Th17 cell balance in a bleomycin-induced scleroderma model. J Immunol. 2010;185(4):2502–15.
•• Gonzalez-Tajuelo R, de la Fuente-Fernandez M, Morales-Cano D, Munoz-Callejas A, Gonzalez-Sanchez E, Silvan J, et al. Spontaneous pulmonary hypertension associated with systemic sclerosis in P-selectin glycoprotein ligand 1-deficient mice. Arthritis Rheumatol. 2020;72(3):477-87. This article reveals that Psgl1-/- mice develop pulmonary vascular changes similar to those of pulmonary arterial hypertension associated with systemic sclerosis, suggesting that the PSGL-1-dependent axis is involved in the immune tolerance related to this complication.
Silván J, González-Tajuelo R, Vicente-Rabaneda E, Pérez-Frías A, Espartero-Santos M, Muñoz-Callejas A, et al. Deregulated PSGL-1 expression in B cells and dendritic cells may be implicated in human systemic sclerosis development. J Invest Dermatol. 2018;138(10):2123–32.
Urzainqui A, Martínez del Hoyo G, Lamana A, de la Fuente H, Barreiro O, Olazabal IM, et al. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J Immunol. 2007;179(11):7457–65.
Bourji K, Meyer A, Chatelus E, Pincemail J, Pigatto E, Defraigne JO, et al. High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis. Free Radic Biol Med. 2015;87:282–9.
Vona R, Giovannetti A, Gambardella L, Malorni W, Pietraforte D, Straface E. Oxidative stress in the pathogenesis of systemic scleroderma: An overview. J Cell Mol Med. 2018;22(7):3308–14.
Piera-Velazquez S, Makul A, Jimenez SA. Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor beta. Arthritis Rheumatol. 2015;67(10):2749–58.
McHugh NJ, Whyte J, Harvey G, Haustein UF. Anti-topoisomerase I antibodies in silica-associated systemic sclerosis. A model for autoimmunity. Arthritis Rheum. 1994;37(8):1198–205.
Herrick AL, Matucci CM. The emerging problem of oxidative stress and the role of antioxidants in systemic sclerosis. Clin Exp Rheumatol. 2001;19(1):4–8.
Servettaz A, Goulvestre C, Kavian N, Nicco C, Guilpain P, Chéreau C, et al. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J Immunol. 2009;182(9):5855–64.
Bagnato G, Bitto A, Pizzino G, Roberts WN, Squadrito F, Altavilla D, et al. Propylthiouracil modulates aortic vasculopathy in the oxidative stress model of systemic sclerosis. Vascul Pharmacol. 2015;71:79–83.
Bitto A, Bagnato GL, Pizzino G, Roberts WN, Irrera N, Minutoli L, et al. Simvastatin prevents vascular complications in the chronic reactive oxygen species murine model of systemic sclerosis. Free Radic Res. 2016;50(5):514–22.
Raker VK, Ook KY, Haub J, Lorenz N, Schmidt T, Stegemann A, et al. Myeloid cell populations and fibrogenic parameters in bleomycin- and HOCl-induced fibrosis. Exp Dermatol. 2016;25(11):887–94.
Sanges S, Jendoubi M, Kavian N, Hauspie C, Speca S, Crave JC, et al. B Cell Homeostasis and Functional Properties Are Altered in an Hypochlorous Acid-Induced Murine Model of Systemic Sclerosis. Front Immunol. 2017;8:53.
Maria ATJ, Toupet K, Maumus M, Rozier P, Vozenin MC, Le Quellec A, et al. Fibrosis development in HOCl-induced systemic sclerosis: a multistage process hampered by mesenchymal stem cells. Front Immunol. 2018;9:2571.
Arnett FC, Cho M, Chatterjee S, Aguilar MB, Reveille JD, Mayes MD. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis Rheum. 2001;44(6):1359–62.
Feghali-Bostwick C, Medsger TA Jr, Wright TM. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum. 2003;48(7):1956–63.
Broen JC, Radstake TR, Rossato M. The role of genetics and epigenetics in the pathogenesis of systemic sclerosis. Nat Rev Rheumatol. 2014;10(11):671–81.
Sharif R, Mayes MD, Tan FK, Gorlova OY, Hummers LK, Shah AA, et al. IRF5 polymorphism predicts prognosis in patients with systemic sclerosis. Ann Rheum Dis. 2012;71(7):1197–202.
Lafyatis R, O’Hara C, Feghali-Bostwick CA, Matteson E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 2007;56(9):3167–8.
Fujimoto M, Sato S. B lymphocytes and systemic sclerosis. Curr Opin Rheumatol. 2005;17(6):746–51.
•• Nakamura K, Taniguchi T, Hirabayashi M, Yamashita T, Saigusa R, Miura S, et al. Altered properties of endothelial cells and mesenchymal stem cells underlying the development of scleroderma-like vasculopathy in KLF5+/-;Fli-1+/- mice. Arthritis Rheumatol. 2020;72(12):2136-46. This study demonstrates that the simultaneous deficiency of KLF5 and FLI1 induces SSc-like features in dermal microvascular endothelial cells, bone marrow-derived endothelial progenitor cells, and bone marrow-derived mesenchymal stem cells, a precursor of pericytes, helping us further understand the developmental process of SSc vasculopathy based on dysregulated angiogenesis and defective vasculogenesis.
Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50(12):3985–93.
Matucci-Cerinic M, Denton CP, Furst DE, Mayes MD, Hsu VM, Carpentier P, et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2011;70(1):32–8.
Saigusa R, Asano Y, Yamashita T, Taniguchi T, Takahashi T, Ichimura Y, et al. Fli1 deficiency contributes to the downregulation of endothelial protein C receptor in systemic sclerosis: a possible role in prothrombotic conditions. Br J Dermatol. 2016;174(2):338–47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Yoshihid Asano declares that no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Asano, Y. Insights Into the Preclinical Models of SSc. Curr Treat Options in Rheum 7, 334–348 (2021). https://doi.org/10.1007/s40674-021-00187-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-021-00187-w