Abstract
Purpose of review
Advances in genetic techniques have led to the discovery of new autoinflammatory syndromes in the last three years. The purpose of this review is to discuss novel autoinflammatory syndromes including disease mechanisms, characteristics, and treatments. We will also discuss recent findings regarding the pathophysiology of well-known autoinflammatory disorders.
Recent findings
Several recently described autoinflammatory disorders will be discussed including NLRC4-MAS, NAIAD, CANDLE, SAVI, ORAS, HA20, DADA2, and APLAID. There is now a better understanding of the pathophysiology of autoinflammatory disorders that has allowed for categorization of these disorders into interferonopathies, inflammasome mediated, or NF-κB dysregulated.
Summary
With a better understanding of these disorders, targeted treatments based on their mechanism are now possible. Some of these disorders have an unknown mechanism of action, and novel disorders are likely. Further research on autoinflammatory disorders is necessary to improve the outcomes of these patients.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Turvey SE, Broide DH. Innate immunity. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S24–32. https://doi.org/10.1016/j.jaci.2009.07.016.
Medzhitov R, Janeway CA Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91(3):295–8. https://doi.org/10.1016/S0092-8674(00)80412-2.
Volpi S, Picco P, Caorsi R, Candotti F, Gattorno M. Type I interferonopathies in pediatric rheumatology. Pediatr Rheumatol Online J. 2016;14(1):35. https://doi.org/10.1186/s12969-016-0094-4.
Verbsky JW. When to suspect autoinflammatory/recurrent fever syndromes. Pediatr Clin N Am. 2017;64(1):111–25. https://doi.org/10.1016/j.pcl.2016.08.008.
de Torre-Minguela C, Mesa Del Castillo P, Pelegrin P. The NLRP3 and pyrin inflammasomes: implications in the pathophysiology of autoinflammatory diseases. Front Immunol. 2017;8:43.
Sag E, Bilginer Y, Ozen S. Autoinflammatory diseases with periodic fevers. Curr Rheumatol Rep. 2017;19(7):41. https://doi.org/10.1007/s11926-017-0670-8.
Kone-Paut I, Quartier P, Fain O, Grateau G, Pillet P, le Blay P, et al. Real-world experience and impact of canakinumab in cryopyrin-associated periodic syndrome: results from a French observational study. Arthritis Care Res. 2017;69(6):903–11. https://doi.org/10.1002/acr.23083.
Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis Rheum. 2012;64(7):2375–86. https://doi.org/10.1002/art.34409.
Cekin N, Akyurek ME, Pinarbasi E, Ozen F. MEFV mutations and their relation to major clinical symptoms of familial Mediterranean fever. Gene. 2017;626:9–13. https://doi.org/10.1016/j.gene.2017.05.013.
Manthiram K, Zhou Q, Aksentijevich I, Kastner DL. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat Immunol. 2017;18(8):832–42. https://doi.org/10.1038/ni.3777.
Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17(8):914–21. https://doi.org/10.1038/ni.3457.
Campbell, L., Raheem I., Malemud C., Askari A., The relationship between NALP3 and autoinflammatory syndromes. Int J Mol Sci. 2016;17(5):725. https://doi.org/10.3390/ijms17050725.
Omenetti A, Carta S, Caorsi R, Finetti M, Marotto D, Lattanzi B, et al. Disease activity accounts for long-term efficacy of IL-1 blockers in pyogenic sterile arthritis pyoderma gangrenosum and severe acne syndrome. Rheumatology (Oxford). 2016;55(7):1325–35. https://doi.org/10.1093/rheumatology/kew031.
Raghawan AK, Sripada A, Gopinath G, Pushpanjali P, Kumar Y, Radha V, et al. A disease-associated mutant of NLRC4 shows enhanced interaction with SUG1 leading to constitutive FADD-dependent caspase-8 activation and cell death. J Biol Chem. 2017;292(4):1218–30. https://doi.org/10.1074/jbc.M116.763979.
Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46(10):1140–6. https://doi.org/10.1038/ng.3089.
Aksentijevich I. Update on genetics and pathogenesis of autoinflammatory diseases: the last 2 years. Semin Immunopathol. 2015;37(4):395–401. https://doi.org/10.1007/s00281-015-0478-4.
Volker-Touw CM, et al. Erythematous nodes, urticarial rash and arthralgias in a large pedigree with NLRC4-related autoinflammatory disease, expansion of the phenotype. Br J Dermatol. 2017;176(1):244–8. https://doi.org/10.1111/bjd.14757.
Vance RE. The NAIP/NLRC4 inflammasomes. Curr Opin Immunol. 2015;32:84–9. https://doi.org/10.1016/j.coi.2015.01.010.
• Canna SW, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139(5):1698–701. This paper provides evidence for the possibility of treating IL-1 inhibitor-resistant NLRC4-associated diseases with IL-18 inhibitors.
Chavarria-Smith J, Vance RE. The NLRP1 inflammasomes. Immunol Rev. 2015;265(1):22–34. https://doi.org/10.1111/imr.12283.
Zhong FL, Mamaï O, Sborgi L, Boussofara L, Hopkins R, Robinson K, et al. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell. 2016;167(1):187–202.e17. https://doi.org/10.1016/j.cell.2016.09.001.
• Grandemange S, et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann Rheum Dis. 2017;76(7):1191–8. This study describes a new autoinflammatory syndrome known as NAIAD as well as successful treatment with IL-1 inhibitors.
Cox AJ, Darbro BW, Laxer RM, Velez G, Bing X, Finer AL, et al. Recessive coding and regulatory mutations in FBLIM1 underlie the pathogenesis of chronic recurrent multifocal osteomyelitis (CRMO). PLoS One. 2017;12(3):e0169687. https://doi.org/10.1371/journal.pone.0169687.
Bader-Meunier B, van Nieuwenhove E, Breton S, Wouters C. Bone involvement in monogenic autoinflammatory syndromes. Rheumatology (Oxford). 2017; https://doi.org/10.1093/rheumatology/kex306.
Cox AJ, Zhao Y, Ferguson PJ. Chronic recurrent multifocal osteomyelitis and related diseases-update on pathogenesis. Curr Rheumatol Rep. 2017;19(4):18. https://doi.org/10.1007/s11926-017-0645-9.
Ulusoy E, Karaca NE, el-Shanti H, Kilicoglu E, Aksu G, Kutukculer N. Interleukin-1 receptor antagonist deficiency with a novel mutation; late onset and successful treatment with canakinumab: a case report. J Med Case Rep. 2015;9(1):145. https://doi.org/10.1186/s13256-015-0618-4.
Rodero MP, Crow YJ. Type I interferon-mediated monogenic autoinflammation: the type I interferonopathies, a conceptual overview. J Exp Med. 2016;213(12):2527–38. https://doi.org/10.1084/jem.20161596.
Kim H, Sanchez GA, Goldbach-Mansky R. Insights from Mendelian interferonopathies: comparison of CANDLE, SAVI with AGS, monogenic lupus. J Mol Med. 2016;94(10):1111–27. https://doi.org/10.1007/s00109-016-1465-5.
•• Torrelo A. CANDLE syndrome as a paradigm of proteasome-related autoinflammation. Front Immunol. 2017;8:927. This review article discusses the characteristics and mechanism of the new autoinflammatory syndrome CANDLE. It also briefly discusses the possibility of the use of JAK inhibitors in the treatment of the syndrome which is currently under investigation.
Kitamura A, Maekawa Y, Uehara H, Izumi K, Kawachi I, Nishizawa M, et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest. 2011;121(10):4150–60. https://doi.org/10.1172/JCI58414.
•• Brehm A, et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J Clin Invest. 2015;125(11):4196–211. This research article identifies eight novel mutations in four proteasome genes other than PSMB8 that link immunoproteasome dysfunction with increased interferon production which provides insight into the genetics and mechanism of CANDLE syndrome.
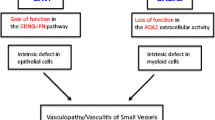
•• Liu Y, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371(6):507–18. This study discovered genetic mutations and mechanisms associated with SAVI. It also provided some of the first evidence of the potential use of JAK inhibitors in the treatment of SAVI.
• Dobbs N, et al. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe. 2015;18(2):157–68. This article helps to provide further evidence for the mechanism and role of STING in autoinflammatory syndromes such as SAVI.
•• Melki I, et al. Disease-associated mutations identify a novel region in human STING necessary for the control of type I interferon signaling. J Allergy Clin Immunol. 2017;140(2):543–552.e5. This study identified three novel mutations in the STING protein that affect the regulation of type 1 interferon production which can provide further insight into the pathology of SAVI. The study also provided evidence for the possible benefit of JAK inhibitors in the treatment of SAVI.
Liu Y, Ramot Y, Torrelo A, Paller AS, Si N, Babay S, et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2012;64(3):895–907. https://doi.org/10.1002/art.33368.
Cavalcante MP, et al. CANDLE syndrome: chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature-a rare case with a novel mutation. Eur J Pediatr. 2016;175(5):735–40. https://doi.org/10.1007/s00431-015-2668-4.
McDermott A, Jacks J, Kessler M, Emanuel PD, Gao L. Proteasome-associated autoinflammatory syndromes: advances in pathogeneses, clinical presentations, diagnosis, and management. Int J Dermatol. 2015;54(2):121–9. https://doi.org/10.1111/ijd.12695.
• Fremond ML, et al. Efficacy of the Janus kinase 1/2 inhibitor ruxolitinib in the treatment of vasculopathy associated with TMEM173-activating mutations in 3 children. J Allergy Clin Immunol. 2016;138(6):1752–5. https://doi.org/10.1016/j.jaci.2016.07.015. This article provides evidence for the benefit of JAK inhibitors in the treatment of SAVI.
Wu X, Yang J, Na T, Zhang K, Davidoff AM, Yuan BZ, et al. RIG-I and IL-6 are negative-feedback regulators of STING induced by double-stranded DNA. PLoS One. 2017;12(8):e0182961. https://doi.org/10.1371/journal.pone.0182961.
•• Damgaard RB, et al. The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell. 2016;166(5):1215–1230.e20. This article describes the importance of the deubiquitinase OTULIN in maintaining immune homeostasis. It also describes the association between mutations in OTULIN and the autoinflammatory syndrome ORAS as well as possible treatments.
Lork M, Verhelst K, Beyaert R. CYLD, A20 and OTULIN deubiquitinases in NF-kappaB signaling and cell death: so similar, yet so different. Cell Death Differ. 2017;24(7):1172–83. https://doi.org/10.1038/cdd.2017.46.
Zhou Q, Yu X, Demirkaya E, Deuitch N, Stone D, Tsai WL, et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc Natl Acad Sci U S A. 2016;113(36):10127–32. https://doi.org/10.1073/pnas.1612594113.
•• Zhou Q, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. 2016;48(1):67–73. This study describes mutations in the protein A20, a deubiquitinase, and its mechanism in the new autoinflammatory syndrome haploinsufficiency A20 (HA20).
Zhang M, Peng LL, Wang Y, Wang JS, Liu J, Liu MM, et al. Roles of A20 in autoimmune diseases. Immunol Res. 2016;64(2):337–44. https://doi.org/10.1007/s12026-015-8677-6.
Stoffels M, Kastner DL. Old dogs, new tricks: monogenic autoinflammatory disease unleashed. Annu Rev Genomics Hum Genet. 2016;17(1):245–72. https://doi.org/10.1146/annurev-genom-090413-025334.
• Takagi M, et al. Haploinsufficiency of TNFAIP3 (A20) by germline mutation is involved in autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. 2017;139(6):1914–22. This study discovered a novel mutation in TNFAIP3/A20 that led to an ALPS-like phenotype within a Japanese infant.
Schepp J, Bulashevska A, Mannhardt-Laakmann W, Cao H, Yang F, Seidl M, et al. Deficiency of adenosine deaminase 2 causes antibody deficiency. J Clin Immunol. 2016;36(3):179–86. https://doi.org/10.1007/s10875-016-0245-x.
Caorsi R, Penco F, Grossi A, Insalaco A, Omenetti A, Alessio M, et al. ADA2 deficiency (DADA2) as an unrecognised cause of early onset polyarteritis nodosa and stroke: a multicentre national study. Ann Rheum Dis. 2017;76(10):1648–56. https://doi.org/10.1136/annrheumdis-2016-210802.
Nanthapisal S, Murphy C, Omoyinmi E, Hong Y, Standing A, Berg S, et al. Deficiency of adenosine deaminase type 2: a description of phenotype and genotype in fifteen cases. Arthritis Rheum. 2016;68(9):2314–22. https://doi.org/10.1002/art.39699.
•• Zhou Q, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370(10):911–20. This study was one of the first to describe the new autoinflammatory syndrome ADA2. They discovered the genetic mutation and described the hypothesized underlying disease mechanism.
Elbracht M, Mull M, Wagner N, Kuhl C, Abicht A, Kurth I, et al. Stroke as initial manifestation of adenosine deaminase 2 deficiency. Neuropediatrics. 2017;48(2):111–4. https://doi.org/10.1055/s-0036-1597611.
van Montfrans J, Zavialov A, Zhou Q. Mutant ADA2 in vasculopathies. N Engl J Med. 2014;371(5):478. https://doi.org/10.1056/NEJMc1405506#SA1.
•• Chae JJ, et al. Connecting two pathways through Ca 2+ signaling: NLRP3 inflammasome activation induced by a hypermorphic PLCG2 mutation. Arthritis Rheumatol. 2015;67(2):563–7. This study discusses the new autoinflammatory syndrome APLAID and the role of the NLRP3 inflammasome in its pathogenesis.
Milner JD. PLAID: a syndrome of complex patterns of disease and unique phenotypes. J Clin Immunol. 2015;35(6):527–30. https://doi.org/10.1007/s10875-015-0177-x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Danielle Fair declares that she has no conflict of interest. James Verbsky declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Rheumatology
Rights and permissions
About this article
Cite this article
Fair, D., Verbsky, J. Update on Autoinflammatory Syndromes. Curr Treat Options in Rheum 4, 73–84 (2018). https://doi.org/10.1007/s40674-018-0093-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-018-0093-3