Abstract
Purpose
Since vertebral fragility fractures (VFFs) might increase the risk of subsequent fractures, we evaluated the incidence rate and the refracture risk of subsequent vertebral and non-vertebral fragility fractures (nVFFs) in untreated patients with a previous VFF.
Methods
We systematically searched PubMed, Embase, and Cochrane Library up to February 2022 for randomized clinical trials (RCTs) that analyzed the occurrence of subsequent fractures in untreated patients with prior VFFs. Two authors independently extracted data and appraised the risk of bias in the selected studies. Primary outcomes were subsequent VFFs, while secondary outcomes were further nVFFs. The outcome of refracture within ≥ 2 years after the index fracture was measured as (i) rate, expressed per 100 person-years (PYs), and (ii) risk, expressed in percentage.
Results
Forty RCTs met our inclusion criteria, ranging from medium to high quality. Among untreated patients with prior VFFs, the rate of subsequent VFFs and nVFFs was 12 [95% confidence interval (CI) 9–16] and 6 (95% CI 5–8%) per 100 PYs, respectively. The higher the number of previous VFFs, the higher the incidence. Moreover, the risk of VFFs and nVFFs increased within 2 (16.6% and 8%) and 4 years (35.1% and 17.4%) based on the index VFF.
Conclusion
The highest risk of subsequent VFFs or nVFFs was already detected within 2 years following the initial VFF. Thus, prompt interventions should be designed to improve the detection and treatment of VFFs, aiming to reduce the risk of future FFs and properly implement secondary preventive measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragility fractures, resulting from a low-impact event such as a fall from a standing height, typically affect the elderly and individuals with poor bone quality [1]. The rising aging population in high-income countries is responsible for increasing rates of fragility fractures and their clinical and functional consequences [1, 2]. Indeed, older adults with an osteoporotic fracture are at higher risk of an imminent fracture, which is a subsequent event within 1–2 years after an index fracture [3, 4]. Therefore, secondary prevention should be adopted after an initial fracture to reduce the further risk of an imminent fracture [3]. In this regard, physicians should evaluate the fracture risk using an assessment tool [5] and are strongly encouraged to treat patients immediately after a sentinel event [6]. Thus, for patients with a high fracture risk, pharmacological agents ought to be promptly recommended as they could improve bone mineral density and reduce the incidence of subsequent fractures [4, 7].
Specifically, vertebral fragility fractures (VFFs), which are among the most common fragility fractures, (i) are the primary risk factor for the occurrence of further fractures affecting either vertebra or other sites [8,9,10,11], (ii) are associated with an increased risk of morbidity and mortality, and (iii) represent a significant economic burden on healthcare systems [12,13,14,15,16]. Although at least one in five persons aged > 50 years has ≥ 1 vertebral fracture [9, 16], detection of VFFs may be uneasy due to ambiguous terminology and the lack of diagnostic standards [2, 11, 14, 15, 17, 18]. Only a third of VFFs come to medical attention [16, 19], which might lead to inadequate patient care [18].
The current meta-analysis systematically reviewed randomized clinical trials (RCTs) investigating the efficacy of drugs for secondary prevention of refracture among patients who experienced vertebral fractures. We summarized data of patients who were randomly assigned to the control arm (i.e., who did not receive drugs for bone fragility care except calcium and vitamin D supplements), which are known to be ineffective for preventing fractures in community-dwelling adults [20]. Thus, we considered patients who received placebo as a proxy of patients with unrecognized fragility fractures. The aim of this systematic review and meta-analysis was to measure the implications of vertebral fractures on the risk of a new fracture in patients with unidentified frailty.
Methods
Search strategy and selection criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [21] for conducting and reporting this study. We did a systematic search of the Embase, PubMed (Medline), and Cochrane Library databases to cover primary studies, as well as systematic reviews published up to February 2022. A hand-checking search on clinicaltrial.gov was performed to detect additional eligible studies. The search strategy included keywords and/or corresponding MeSH terms related to “vertebral fragility fracture” and “subsequent fragility fractures”. Further details and search terms are listed in Supplemental Material.
Studies were eligible if they (i) were RTCs, (ii) reported data on refracture following a radiographically detected index fracture (or morphometric fracture) among (iii) patients with a VFF who, being randomly assigned to the comparator arm, did not have drug treatment for bone fragility. Vertebral and non-vertebral refracture occurring at the time point following the index fracture were considered primary and secondary outcomes, respectively. Studies were excluded if they (i) were not published in the English language, (ii) did not report original findings (i.e., letters and case reports), (iii) did not involve patients with at least one VFF at baseline, or (iv) did not evaluate the refracture risk. When data were published more than once, the most recent and complete paper was selected. Besides, if multiple articles were published on the same trial, all articles reporting different follow-up periods or different refracture sites were included.
Two independent authors (GP and AB) screened titles and abstracts according to the search strategy and then assessed the full text of all potentially relevant studies. Discrepancies between readers were resolved by conference. From each included RCT, the following information was extracted: (i) first author, year, and country of publication; (ii) type and characteristics of the target population; (iii) type of refracture; (iv) follow-up period.
Study quality
The quality of each RCT was evaluated using the Cochrane risk of bias (RoB) tool for RCTs [22]. The following domains of the Cochrane RoB tool were appraised: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias (such as funding bias). Each domain was classified as “high” or “low” RoB. The latter was considered “unclear” if the publication did not provide sufficient information. The overall quality of each included study was judged as high, medium, or low if no high (and fewer than three unclear), at most one high (or more than three unclear), or more than one high RoB was found, respectively.
Statistical analysis
Only patients belonging to the comparator arm for whom a radiograph exam was performed to clinically recognize the index fracture (i.e., fractured patients at risk of developing further fractures) were considered in the current meta-analysis.
The refracture outcome was measured through both rate and risk. The refracture rate was calculated as the number of patients assigned to the comparator arm who experienced a subsequent bone fracture over the person-years (PYs) from them accumulated. The refracture rate was expressed as per 100 PYs of follow-up and was presented with 95% confidence intervals (CIs). Unless directly reported in the original publication, PYs were derived as years accumulated during follow-up by patients at risk of developing the outcome (by right censuring observations at outcome occurrence or lost to follow-up when feasible). The refracture risk was calculated as the number of patients assigned to the comparator arm who experienced the outcome within two, three, and four years over the number of patients randomized to the comparator arm and expressed as a percentage with corresponding 95% CIs.
Estimates were summarized if at least three studies reported the estimate of interest. In the case of < 3 studies per category, data were aggregated into larger classes.
Subgroup analyses were planned for (i) the baseline number of VFFs and (ii) specific sites of no vertebral fracture during follow-up.
Heterogeneity between study-specific estimates was tested using Chi-square statistics [23] and measured with the I2 index (heterogeneity measure across studies) [24]. Studies were combined to obtain a summary estimate using the DerSimonian random-effects model [25]. Potential publication bias was visually and statistically identified through funnel plots and Egger’s test [26]. Furthermore, influence analysis was performed to assess the impact of a single study on the overall pooled estimates by omitting one study at a time.
All tests were considered statistically significant for p-values < 0.05. The analyses and the correspondent graphical visualization of forest plots were performed using RevMan V.5.4 (Nordic Cochrane Center, Copenhagen, Denmark) and R Statistical Software (v4.1.2; R Core Team 2021).
Results
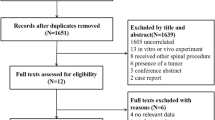
As shown in Fig. 1, a total of 1184 papers were initially extracted. Overall, after exclusion through title and abstract screening and further inclusion through papers referenced by systematic reviews [17, 27,28,29,30,31] and hand search on the topic [32], a total of 40 RCTs were included [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]. Summary characteristics of the 40 RCTs included in our meta-analysis are given in Table 1. Twenty-five and fifteen trials were classified into the categories of high or medium quality, respectively. Almost all RCTs had at least one unclear risk of bias, primarily regarding selection bias (random sequence generation or allocation concealment tools) or other bias. Moreover, 14 RCTs [34, 38, 40, 42, 44, 45, 48, 49, 55, 59,60,61, 63, 68] had a high risk of bias, mainly due to attrition (incomplete outcome data) (Supplemental Material, Figure S1–S2). Overall, 9,891 patients with at least one baseline VFF who did not receive drug treatment for correcting bone fragility except for supplements with calcium and/or vitamin D were considered to be summarized in our meta-analysis. These patients accumulated 22,990 PYs for the risk of refracture. Then, the majority of the included studies were focused on post-menopausal women.
Figure 2 shows the forest plots of vertebral and non-vertebral refracture rates. Vertebral fracture rates ranged from 3 to 73 refractures per 100 PYs, being an overall estimate of 12 (95% CI 9–16) refractures per 100 PYs and having very high between-studies heterogeneity (I2 ≥ 90%) (Fig. 2, upper box). During the follow-up period, an increase in the rate of vertebral fractures was observed, specifically 4 (2–7) per 100 PY for those with 1 fracture at baseline and 13 (6–29) per 100 PY for those with more than 2 fractures at baseline (Supplementary Material, Figure S3). nVFFs rates ranged from 2 to 19 refractures per 100 PYs, being the overall estimate of 6 (95% CI: 5–8) refractures per 100 PYs and having high between-studies heterogeneity (I2 ≥ 75%) (Fig. 2, bottom box).
The summary fracture rate of (i) upper limbs was 1.3 (95% CI 1.0–1.7), 2.5 (0.7–9.4), and 0.8 (0.3–2.0) per 100 PYs for wrist, arm or forearm, and humerus, respectively (Supplemental Material, Figure S4); (ii) lower limbs was 0.9 (0.7–1.2), 0.4 (0.2–0.6), 0.4 (0.2–0.6), and 0.8 (0.4–1.7) per 100 PYs for hip, ankle, pelvis, foot or metatarsal, respectively (Supplemental Material, Figure S5); (iii) torso was 1.4 (0.5–4.1) (Supplemental Material, Figure S6); and (iv) other fractures was 2.6 (1.6–4.3) (Supplemental Material, Figure S7). The between-studies heterogeneity was reduced for upper and lower limb fractures (I2 < 75%).
Publication bias was detected for vertebral fractures (p-value = 0.0124, Supplemental Material, Figures S8–S9), although there was no evidence of the influence of any individual study (Supplemental Material, Figure S10) for both vertebral and non-vertebral refractures.
Figures 3 and 4 depict the forest plots of vertebral and non-vertebral refracture risks, respectively. Within two, three, and four years from the index fracture, 16.6% (13.1–20.8%), 25.7% (18.8–34.1%), and 35.1% (24.4–47.7%) of patients, respectively, experienced at least a vertebral refracture, while the non-vertebral fracture risk was 8.0% (4.6–13.4%) within 2 years and 17.4% (14.1–21.4%) over 2 years.
Discussion
In this meta-analysis of 40 original RCTs including almost 10,000 untreated patients affected by vertebral fracture at baseline, we found that, on average, a new vertebral and non-vertebral fracture occurred every year in 12 and 6 patients, respectively, per 100 patients who had previously experienced a vertebral fracture. Our meta-analysis found that 16.6% and 35.1% of patients experienced at least a vertebral refracture within two and four years from the index fracture, respectively, while non-vertebral fracture risk was 8.0% within 2 years and 17.4% over 2 years.
Our results are confirmed by the findings in the literature. Particularly, the UK clinical guideline for the prevention and treatment of osteoporosis revealed a doubled fracture risk related to the prior fracture, particularly for > 1 vertebral fracture [73]. Then, a summary of the literature reported a strong association, approximately 4 times greater, between prior and subsequent vertebral fractures than those without prior fractures, particularly within the next 2 years after the initial fracture [74]. The risk of further vertebral fractures appeared to increase with the number of prior vertebral fractures [74].
Early identification of VFFs might present a real opportunity to reduce the risk of a subsequent fracture [75]. However, there is considerable evidence that vertebral fractures might not be properly considered by clinicians and are under-reported by radiologists [14, 76], who might not use specific terminology and not alert the referring healthcare professionals. Thus, standardized radiographic acquisition and unambiguous radiological interpretation could contribute to reducing the further risk of VFFs [76].
Because the patients included in our meta-analysis received a drug therapy that should be considered ineffective for the treatment of fragility, all these findings strongly suggest that recognizing fragility as the cause or concomitant cause of the vertebral fracture should be considered a priority for the secondary prevention of fracture.
Strengths and limitations
The findings of this study should be interpreted considering its limitations. First, the analysis was not patient-centered but instead used summary data; therefore, an accurate assessment might be lacking due to the nature of meta-analysis. Second, this systematic review selected RCTs that included patients who might have different characteristics compared to the general population. Third, our results were affected by high heterogeneity. Particularly, there are certain concerns as to whether findings from selected studies could be combined into one conclusion since primary findings were obtained from studies including heterogeneous populations, definitions of vertebral fractures and adopting different study designs. Fourth, among trials in which PYs were not reported, we assumed that censorships occurred in a mean of half of the entire follow-up period and could estimate below or above the incidence rate. However, this assumption can reasonably be considered valid in the case of large data and/or time intervals of limited amplitude. At last, an unclear risk of bias was found in nearly all the included studies, primarily regarding attrition bias, and a high risk of bias was detected in 12 RCTs, mainly due to attrition bias.
Despite these limitations, this study had certain strengths. The exhaustive search strategy provided an overview of RCTs on the subsequent fractures among untreated patients with prior VFFs. In addition, the internal validity of the selected studies was assessed using the RoB tool for RCTs.
Conclusion
The average annual rate and short-long term risk of refracture among untreated patients with VFFs were estimated from this meta-analysis. Based upon the currently available evidence, further fractures are commonly observed in the following two years after the initial VFF. Early and accurate detection of VFFs should be conducted to reduce the risk of future fragility fractures and properly establish secondary prevention.
Data availability
No additional data is available.
References
Dang DY, Zetumer S, Zhang AL (2019) Recurrent fragility fractures: a cross-sectional analysis. J Am Acad Orthop Surg 27:e85-91
Capdevila-Reniu A, Navarro-López M, López-Soto A (2019) Osteoporotic vertebral fractures: a diagnostic challenge in the 21st century. Rev Clin Esp S0014–2565(19):30240–30241
Wong RMY, Wong PY, Liu C, Wong HW, Chung YL, Chow SKH et al (2022) The imminent risk of a fracture-existing worldwide data: a systematic review and meta-analysis. Osteoporos Int 33:2453–2466
Liu J, Curtis EM, Cooper C, Harvey NC (2019) State of the art in osteoporosis risk assessment and treatment. J Endocrinol Invest 42:1149–1164
Adami G, Biffi A, Porcu G, Ronco R, Alvaro R, Bogini R et al (2023) A systematic review on the performance of fracture risk assessment tools: FRAX, DeFRA FRA-HS. J Endocrinol Invest. https://doi.org/10.1007/s40618-023-02082-8
Johansson H, Siggeirsdóttir K, Harvey NC, Odén A, Gudnason V, McCloskey E et al (2017) Imminent risk of fracture after fracture. Osteoporos Int 28:775–780
Luo C, Qin SX, Wang QY, Li YF, Qu XL, Yue C et al (2023) Cost-effectiveness analysis of five drugs for treating postmenopausal women in the United States with osteoporosis and a very high fracture risk. J Endocrinol Invest 46:367–379
Balasubramanian A, Zhang J, Chen L, Wenkert D, Daigle SG, Grauer A et al (2019) Risk of subsequent fracture after prior fracture among older women. Osteoporos Int 30:79–92
Barton DW, Behrend CJ, Carmouche JJ (2019) Rates of osteoporosis screening and treatment following vertebral fracture. Spine J 19:411–417
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
McDonald CL, Alsoof D, Daniels AH (2013) Vertebral compression fractures. R I Med J 2022(105):40–45
Robinson WA, Carlson BC, Poppendeck H, Wanderman NR, Bunta AD, Murphy S et al (2020) Osteoporosis-related vertebral fragility fractures: a review and analysis of the American Orthopaedic Association’s own the bone database. Spine 45:E430–E438
Bottai V, Giannotti S, Raffaetà G, Mazzantini M, Casella F, De Paola G et al (2016) Underdiagnosis of osteoporotic vertebral fractures in patients with fragility fractures: retrospective analysis of over 300 patients. Clin Cases Miner Bone Metab 13:119–122
Howlett DC, Drinkwater KJ, Griffin J, Javaid K (2020) Improving outcomes for patients with osteoporotic vertebral fragility fractures: the role of the radiologist. Clin Radiol 75:811–812
Alsoof D, Anderson G, McDonald CL, Basques B, Kuris E, Daniels AH (2022) Diagnosis and management of vertebral compression fracture. Am J Med 135:815–821
Zileli M, Fornari M, Costa F, Anania CD, Parthiban J, Sharif S (2022) Epidemiology, natural course, and preventive measures of osteoporotic vertebral fractures: WFNS Spine Committee Recommendations. J Neurosurg Sci 66:282–290
Zeytinoglu M, Jain RK, Vokes TJ (2017) Vertebral fracture assessment: enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone 104:54–65
Borges JLC, Maia JL, Silva RF, Lewiecki EM (2015) Diagnosing vertebral fractures: missed opportunities. Rev Bras Reumatol 55:464–467
Lems WF, Paccou J, Zhang J, Fuggle NR, Chandran M, Harvey NC et al (2021) Vertebral fracture: epidemiology, impact and use of DXA vertebral fracture assessment in fracture liaison services. Osteoporos Int 32:399–411
Reid IR, Bolland MJ (2020) Calcium and/or vitamin D supplementation for the prevention of fragility fractures: who needs it? Nutrients 12:1011
Page MJ, Moher D (2017) Evaluations of the uptake and impact of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and extensions: a scoping review. Syst Rev 6:263
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129. https://doi.org/10.2307/3001666
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Lippuner K (2003) Medical treatment of vertebral osteoporosis. Eur Spine J 12(Suppl 2):S132-141
Boonen S, Laan RF, Barton IP, Watts NB (2005) Effect of osteoporosis treatments on risk of non-vertebral fractures: review and meta-analysis of intention-to-treat studies. Osteoporos Int 16:1291–1298
Francis RM, Anderson FH, Torgerson DJ (1995) A comparison of the effectiveness and cost of treatment for vertebral fractures in women. Br J Rheumatol 34:1167–1171
Seeman E, Crans GG, Diez-Perez A, Pinette KV, Delmas PD (2006) Anti-vertebral fracture efficacy of raloxifene: a meta-analysis. Osteoporos Int 17:313–316
Kobayashi T, Kaneko M, Narukawa M (2020) Influence of prevalent vertebral fracture on the correlation between change in lumbar spine bone mineral density and risk of new vertebral fracture: a meta-analysis of randomized clinical trials. Clin Drug Investig 40:15–23
Ensrud KE, Black DM, Palermo L, Bauer DC, Barrett-Connor E, Quandt SA et al (1997) Treatment with alendronate prevents fractures in women at highest risk: results from the fracture intervention trial. Arch Intern Med 157:2617–2624
Sontag A, Wan X, Krege JH (2010) Benefits and risks of raloxifene by vertebral fracture status. Curr Med Res Opin 26:71–76
Meunier PJ, Roux C, Ortolani S, Diaz-Curiel M, Compston J, Marquis P et al (2009) Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporos Int 20:1663–1673
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Prevrhal S, Krege JH, Chen P, Genant H, Black DM (2009) Teriparatide vertebral fracture risk reduction determined by quantitative and qualitative radiographic assessment. Curr Med Res Opin 25:921–928
Nakano T, Shiraki M, Sugimoto T, Kishimoto H, Ito M, Fukunaga M et al (2014) Once-weekly teriparatide reduces the risk of vertebral fracture in patients with various fracture risks: subgroup analysis of the teriparatide once-weekly efficacy research (TOWER) trial. J Bone Miner Metab 32:441–446
Wustrack R, Seeman E, Bucci-Rechtweg C, Burch S, Palermo L, Black DM (2012) Predictors of new and severe vertebral fractures: results from the HORIZON pivotal fracture trial. Osteoporos Int 23:53–58
Meunier PJ, Sebert JL, Reginster JY, Briancon D, Appelboom T, Netter P et al (1998) Fluoride salts are no better at preventing new vertebral fractures than calcium-vitamin D in postmenopausal osteoporosis: the FAVOStudy. Osteoporos Int 8:4–12
Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR et al (2007) Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 146:326–339
McCloskey E, Selby P, Davies M, Robinson J, Francis RM, Adams J et al (2004) Clodronate reduces vertebral fracture risk in women with postmenopausal or secondary osteoporosis: results of a double-blind, placebo-controlled 3-year study. J Bone Miner Res 19:728–736
Montessori ML, Scheele WH, Netelenbos JC, Kerkhoff JF, Bakker K (1997) The use of etidronate and calcium versus calcium alone in the treatment of postmenopausal osteopenia: results of three years of treatment. Osteoporos Int 7:52–58
Brumsen C, Papapoulos SE, Lips P, Geelhoed-Duijvestijn PHLM, Hamdy NAT, Landman JO et al (2002) Daily oral pamidronate in women and men with osteoporosis: a 3-year randomized placebo-controlled clinical trial with a 2-year open extension. J Bone Miner Res 17:1057–1064
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K et al (2009) Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int 20:1429–1437
Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Ohashi Y et al (2013) Number and severity of prevalent vertebral fractures and the risk of subsequent vertebral fractures in Japanese women with osteoporosis: results from the Minodronate trial. J Bone Miner Metab 31:544–550
Clemmesen B, Ravn P, Zegels B, Taquet AN, Christiansen C, Reginster JY (1997) A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int 7:488–495
Fujita T, Fukunaga M, Itabashi A, Tsutani K, Nakamura T (2014) Once-weekly injection of low-dose teriparatide (28.2 μg) reduced the risk of vertebral fracture in patients with primary osteoporosis. Calcif Tissue Int 94:170–175
Gutteridge DH, Stewart GO, Prince RL, Price RI, Retallack RW, Dhaliwal SS et al (2002) A randomized trial of sodium fluoride (60 mg) +/- estrogen in postmenopausal osteoporotic vertebral fractures: increased vertebral fractures and peripheral bone loss with sodium fluoride; concurrent estrogen prevents peripheral loss, but not vertebral fractures. Osteoporos Int 13:158–170
Wimalawansa SJ (1998) A four-year randomized controlled trial of hormone replacement and bisphosphonate, alone or in combination, in women with postmenopausal osteoporosis. Am J Med 104:219–226
Chesnut CH, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A et al (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Felsenberg D, Miller P, Armbrecht G, Wilson K, Schimmer RC, Papapoulos SE (2005) Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2, and 3 years in postmenopausal women with osteoporosis. Bone 37:651–654
Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Törring O et al (2011) Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 96:1727–1736
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Interv Trial Res Group Lancet 348:1535–1541
Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E et al (2003) Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 33:522–532
Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350:459–468
Chesnut CH, Silverman S, Andriano K, Genant H, Gimona A, Harris S et al (2000) A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study PROOF study group. Am J Med 109:267–276
Recker RR, Hinders S, Davies KM, Heaney RP, Stegman MR, Lappe JM et al (1996) Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 11:1961–1966
Aloia JF, Vaswani A, Yeh JK, Ellis K, Yasumura S, Cohn SH (1988) Calcitriol in the treatment of postmenopausal osteoporosis. Am J Med 84:401–408
Gallagher JC, Goldgar D (1990) Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Ann Intern Med 113:649–655
Ott SM, Chesnut CH (1989) Calcitriol treatment is not effective in postmenopausal osteoporosis. Ann Intern Med 110:267–274
Pak CY, Sakhaee K, Adams-Huet B, Piziak V, Peterson RD, Poindexter JR (1995) Treatment of postmenopausal osteoporosis with slow-release sodium fluoride. Final report of a randomized controlled trial. Ann Intern Med 123:401–408
Ringe JD, Kipshoven C, Cöster A, Umbach R (1999) Therapy of established postmenopausal osteoporosis with monofluorophosphate plus calcium: dose-related effects on bone density and fracture rate. Osteoporos Int 9:171–178
Tilyard MW, Spears GF, Thomson J, Dovey S (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 326:357–362
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip intervention program study group. N Engl J Med 344:333–340
Kleerekoper M, Peterson EL, Nelson DA, Phillips E, Schork MA, Tilley BC et al (1991) A randomized trial of sodium fluoride as a treatment for postmenopausal osteoporosis. Osteoporos Int 1:155–161
Lufkin EG, Wahner HW, O’Fallon WM, Hodgson SF, Kotowicz MA, Lane AW et al (1992) Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med 117:1–9
Riggs BL, Hodgson SF, O’Fallon WM, Chao EY, Wahner HW, Muhs JM et al (1990) Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 322:802–809
Storm T, Thamsborg G, Steiniche T, Genant HK, Sørensen OH (1990) Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med 322:1265–1271
Watts NB, Harris ST, Genant HK, Wasnich RD, Miller PD, Jackson RD et al (1990) Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med 323:73–79
Lufkin EG, Whitaker MD, Nickelsen T, Argueta R, Caplan RH, Knickerbocker RK et al (1998) Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res 13:1747–1754
Recker R, Stakkestad JA, Chesnut CH, Christiansen C, Skag A, Hoiseth A et al (2004) Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone 34:890–899
Harris ST, Watts NB, Jackson RD, Genant HK, Wasnich RD, Ross P et al (1993) Four-year study of intermittent cyclic etidronate treatment of postmenopausal osteoporosis: three years of blinded therapy followed by one year of open therapy. Am J Med 95:557–567
Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12:43
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Adams J, Clark EM, Clunie G (2017) Clinical guidance for the effective identification of vertebral fractures. National Osteoporosis Society, Bath
Adams JE, Lenchik L, Roux C, Genant HK. Radiological assessment of vertebral fracture. International osteoporosis foundation vertebral fracture initiative resource document part II 2010.
Acknowledgements
We thank Charlesworth Author Services for the English Academic Editing.
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement. The Italian guideline was funded by ALTIS Omnia Pharma Service, which did not affect the content of the document.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GA declares personal fees from Theramex, Amgen, BMS, Lilly, Fresenius Kabi, and Galapagos. LC declares personal fees from UCB Pharma, Abiogen Pharma, Bruno Farmaceutici, Sandoz, Metagenics. DG has received honoraria as consultant for Eli Lilly, Organon, MSD Italia. SG has received honoraria as consultant for UCB Pharma. SM has received honoraria as consultant for UCB, Eli Lilly, Amgen. MLB has received (i) honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB, (ii) grants and/or speaker: Abiogen, Alexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, UCB Pharma, (iii) consultant: Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB Pharma. GC received research support from the European Community (EC), the Italian Agency of Drug (AIFA), and the Italian Ministry for University and Research (MIUR). He took part to a variety of projects that were funded by pharmaceutical companies (i.e., Novartis, GSK, Roche, AMGEN, and BMS). He also received honoraria as member of Advisory Board from Roche. No other potential conflicts of interest relevant to this article were disclosed. MR declares personal fees from Amgen, ABBvie, BMS, Eli Lilly, Galapagos, Menarini, Novartis, Pfizer, Sandoz, Theramex, and UCB outside the submitted work. RM took part to a project funded by Abiogen Pharma. GI received honoraria as speaker by Eli Lilly, Menarini, UCB Pharma. The other authors declare that they have no conflict of interest. All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no conflicts of interest.
Patient and public involvement statement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Porcu, G., Biffi, A., Ronco, R. et al. Refracture following vertebral fragility fracture when bone fragility is not recognized: summarizing findings from comparator arms of randomized clinical trials. J Endocrinol Invest 47, 795–818 (2024). https://doi.org/10.1007/s40618-023-02222-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02222-0