Abstract
Purpose of review
Identifying the culprit drug or drugs in SCARs (Severe Cutaneous Adverse Reactions) is a complex and challenging task. However, it is necessary for the patient to know which drugs to avoid in the future, alternatives for treatment and not to have forbidden for all his life drugs that were not responsible for the reaction. When to use causality algorithms? To which extent are valid in vitro tests? Are they useful in clinical practice or just research techniques? Are skin tests sensitive and safe in all SCARs? When performing them? What about the re-exposure tests? Is there any indication for them? This review gives answers to these questions.
Recent findings
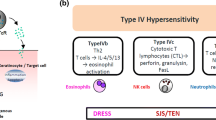
Causality algorithms and the calculation of the index day is the first approach. In vitro tests, LTT (lymphocyte transformation test) is a sensitive and specific test for SJS/TEN (Stevens-Johnson syndrome/toxic epidermal necrolysis) and DRESS (drug reaction with eosinophilia and systemic symptoms) in the recovery phase. ELISpot is useful during the acute phase. Skin tests have different sensitivity according to every particular SCAR and are safe if performing according to guidelines recommendations.
Summary
An approach combining causality algorithms, LTT, and skin tests allow us to identify the culprit drugs or at least limiting the forbidden drugs for the future in a safe way.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Ardern-Jones MR, Mockenhaupt M. Making a diagnosis in severe cutaneous drug hypersensitivity reactions. Curr Opin Allergy Clin Immunol. 2019;19(4):283–93 An updated expert review on diagnosis in SCARs.
Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Chem Immunol Allergy [Internet]. 2012 [cited 2019 May 24];97:1–17. Available from: https://www.karger.com/Article/FullText/335612.
•• Cabañas R, Ramírez E, Sendagorta E, Alamar R, Barranco R, Blanca-López N, et al. Spanish guidelines for diagnosis, management, treatment, and prevention of DRESS syndrome. J Investig Allergol Clin Immunol. 2020;30(4):229–53 First guidelines on practical diagnosis, management, treatment, and prevention on DRESS syndrome published in English. Specifics for causality assessment on DRESS syndrome are detailed.
• Mockenhaupt M. Severe drug-induced skin reactions: clinical pattern, diagnostics and therapy. J Dtsch Dermatol Ges [Internet]. 2009 [cited 2014 Mar 7];7(2):142–60; quiz 161–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19371237. Practical approach on diagnosis of SCARs.
Brockow K, Ardern-Jones MR, Mockenhaupt M, Aberer W, Barbaud A, Caubet JC, et al. EAACI position paper on how to classify cutaneous manifestations of drug hypersensitivity. Allergy Eur J Allergy Clin Immunol. 2019;74(1):14–27.
Mirakian R, Ewan PW, Durham SR, Youlten LJF, Dugué P, Friedmann PS, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy [Internet]. 2009 Jan [cited 2019 May 24];39(1):43–61. Available from: http://doi.wiley.com/10.1111/j.1365-2222.2008.03155.x.
• Creamer D, Walsh SA, Dziewulski P, Exton LS, Lee HY, Dart JKG, et al. U.K. guidelines for the management of Stevens–Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol. 2016;174(6):1194–227 Excellent guidelines for the management of SJS/TEN.
Macedo AF, Marques FB, Ribeiro CF. Can decisional algorithms replace global introspection in the individual causality assessment of spontaneously reported ADRs? Drug Saf [Internet]. 2006 [cited 2019 May 24];29(8):697–702. Available from: http://link.springer.com/10.2165/00002018-200629080-00006.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45.
• Aguirre C, García M. Evaluación de la causalidad en las comunicaciones de reacciones adversas a medicamentos. Algoritmo del Sistema Español de Farmacovigilancia. Med Clin (Barc). 2016;147(10):461–4. https://doi.org/10.1016/j.medcli.2016.06.012. Interesting article where the algorithm of assessment of causality of the Spanish pharmacovigilance system is described. In short, the following seven criteria were scored for each drug, and drug causality was determined by the sum of the scores: (1) the chronology referred to as the interval between drug administration and effect, (2) the literature, defining the degree of knowledge of the relationship between the drug and the effect, (3) the evaluation of drug withdrawal, (4) the rechallenge effect, (5) other cause, (6) risk factor, (7) complementary explorations.
• Cabañas R, Calderón O, Ramírez E, Fiandor A, Caballero T, Heredia R, et al. Sensitivity and specificity of the lymphocyte transformation test in drug reaction with eosinophilia and systemic symptoms causality assessment. Clin Exp Allergy [Internet]. 2018 Mar [cited 2019 May 19];48(3):325–33. Available from: http://doi.wiley.com/10.1111/cea.13076. This article shows the results of LTT assays in the largest series of DRESS cases reported up to date including sensitivity and specificity related to the algorithm of the SPS as the reference for drug causality.
•• Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88(1):60–8. https://doi.org/10.1038/clpt.2009.252. This article shows the construction and evaluation of ALDEN algorithm specific for SJS/TEN. Concluding it can be considered a reference tool in SJS/TEN.
Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69(2):187.e1–16; quiz 203-4. https://doi.org/10.1016/j.jaad.2013.05.002.
• Bellón T, Rodríguez-Martín S, Cabañas R, Ramírez E, Lerma V, González-Herrada C, et al. Assessment of drug causality in Stevens-Johnson syndrome/toxic epidermal necrolysis: concordance between lymphocyte transformation test and ALDEN. Allergy Eur J Allergy Clin Immunol. 2020;75(4):956–9 This article shows the results of LTT assays in the largest series of SJS/TEN cases reported up to date including sensitivity and specificity related to ALDEN as the reference for drug causality.
Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol [Internet]. 2013 Nov [cited 2019 May 24];169(5):1071–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23855313.
Um SJ, Lee SK, Kim YH, Kim KH, Son CH, Roh MS, et al. Clinical features of drug-induced hypersensitivity syndrome in 38 patients. J Investig Allergol Clin Immunol [Internet]. 2010 [cited 2019 May 26];20(7):556–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21313995.
Cabañas R, Calderón O, Ramírez E, Fiandor A, Prior N, Caballero T, et al. Piperacillin-induced DRESS: distinguishing features observed in a clinical and allergy study of 8 patients. J Investig Allergol Clin Immunol. 2014;24(6):425–30
Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127(9):1333–8
Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JNB, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157(5):989–96.
Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139(8):683–93.
• Mayorga C, Celik G, Rouzaire P, Whitaker P, Bonadonna P, Rodrigues-Cernadas J, et al. In vitro tests for drug hypersensitivity reactions: an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy Eur J Allergy Clin Immunol [Internet]. 2016 Aug [cited 2019 May 24];71(8):1103–34. Available from: http://doi.wiley.com/10.1111/all.12886. An interesting position paper of ENDA/EAACI Drug Allergy Interest Group on in vitro tests.
Hari Y, Frutig-Schnyder K, Hurni M, Yawalkar N, Zanni MP, Schnyder B, et al. T cell involvement in cutaneous drug eruptions. Clin Exp Allergy [Internet]. 2001 Sep [cited 2019 May 23];31(9):1398–408. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11591190.
Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest [Internet]. 2001;107(11):1433–41. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed5&NEWS=N&AN=2001205027.
Naisbitt DJ, Farrell J, Wong G, Depta JPH, Dodd CC, Hopkins JE, et al. Characterization of drug-specific T cells in lamotrigine hypersensitivity. J Allergy Clin Immunol [Internet]. 2003 Jun [cited 2019 May 24];111(6):1393–403. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12789244.
Wei CY, Chung WH, Huang HW, Chen YT, Hung SI. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012;129(6):1562–1569.e5. https://doi.org/10.1016/j.jaci.2011.12.990.
• Mayorga C, Sanz ML, Gamboa P, Garcia-Aviles MC, Fernandez J, Torres MJ, et al. In vitro methods for diagnosing nonimmediate hypersensitivity reactions to drugs. J Investig Allergol Clin Immunol [Internet]. 2013 [cited 2019 May 24];23(4):213–25. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L369191650%5Cnhttp://www.jiaci.org/issues/vol23issue4/1.pdf%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=10189068&id=doi:&atitle=In+vitro+methods+for+diagnosing+nonimmediate+h. A practical and comprehensive review on in vitro tests.
•• Pichler WJ, Tilch J. Review article The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy [Internet]. 2004 Aug [cited 2019 May 26];59(8):809–20. Available from: http://doi.wiley.com/10.1111/j.1398-9995.2004.00547.x. This manuscript offers a comprehensive guide to the technical aspects of LTT as an in vitro tool in drug allergy diagnosis.
Beeler A, Pichler WJ. In vitro tests of T-cell-mediated drug hypersensitivity. Drug Hypersensitivity. 2007:380–90.
• Porebski G. In vitro assays in severe cutaneous adverse drug reactions: Are they still research tools or diagnostic tests already? Int J Mol Sci [Internet]. 2017 Aug 10 [cited 2019 May 26];18(8):1–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28796198. A thorough systematic review of in vitro diagnostic test for drug causality assessment in SCARs, including tempative calculations of sensitivity and specificity in defined clinical entities.
Kano Y, Hirahara K, Mitsuyama Y, Takahashi R, Shiohara T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: Dependence on its timing and the type of drug eruption. Allergy Eur J Allergy Clin Immunol [Internet]. 2007 Dec [cited 2019 May 24];62(12):1439–44. Available from: http://doi.wiley.com/10.1111/j.1398-9995.2007.01553.x.
Jörg L, Yerly D, Helbling A, Pichler W. The role of drug, dose, and the tolerance/intolerance of new drugs in multiple drug hypersensitivity syndrome. Allergy Eur J Allergy Clin Immunol. 2020;75(5):1178–87.
• Liccioli G, Mori F, Parronchi P, Capone M, Fili L, Barni S, et al. Aetiopathogenesis of severe cutaneous adverse reactions (SCARs) in children: a 9-year experience in a tertiary care paediatric hospital setting. Clin Exp Allergy. 2020;50(1):61–73 This article shows the results of allergy work-up in the largest series of SCAR cases reported in pediatric patients.
Beeler A, Engler O, Gerber BO, Pichler WJ. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol. 2006;117(2):455–62.
Lochmatter P, Beeler A, Kawabata TT, Gerber BO, Pichler WJ. Drug-specific in vitro release of IL-2, IL-5, IL-13 and IFN-gamma in patients with delayed-type drug hypersensitivity. Allergy [Internet]. 2009 Sep [cited 2019 May 24];64(9):1269–78. Available from: http://doi.wiley.com/10.1111/j.1398-9995.2009.01985.x.
Lochmatter P, Zawodniak A, Pichler WJ. In vitro tests in drug hypersensitivity diagnosis. Immunol Allergy Clin North Am [Internet]. 2009 Aug [cited 2019 May 24];29(3):537–54. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0889856109000332.
Martin M, Wurpts G, Ott H, Baron JM, Erdmann S, Merk HF, et al. In vitro detection and characterization of drug hypersensitivity using flow cytometry. Allergy Eur J Allergy Clin Immunol. 2010;65(1):32–9.
Zawodniak A, Lochmatter P, Yerly D, Kawabata T, Lerch M, Yawalkar N, et al. In vitro detection of cytotoxic T and NK cells in peripheral blood of patients with various drug-induced skin diseases. Allergy Eur J Allergy Clin Immunol. 2010;65(3):376–84.
Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome - alternatives for lymphocyte transformation test. Clin Exp Allergy. 2013;43(9):1027–37.
Polak ME, Belgi G, McGuire C, Pickard C, Healy E, Friedmann PS, et al. In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensitivity reactions. Br J Dermatol [Internet]. 2013 Mar [cited 2019 May 26];168(3):539–49. Available from: http://doi.wiley.com/10.1111/bjd.12109.
Chung W-H, Pan R-Y, Chu M-T, Chin S-W, Huang Y-L, Wang W-C, et al. Oxypurinol-specific T Cells possess preferential TCR clonotypes and express granulysin in allopurinol-induced severe cutaneous adverse reactions. J Invest Dermatol [Internet]. 2015 Sep [cited 2019 May 19];135(9):2237–48. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022202X1539014X.
Chen CB. The risk of anti-osteoporotic agent-induced severe cutaneous adverse drug reactions and their association with HLA. J Eur Acad Dermatol Venereol. 2020;7. https://doi.org/10.1111/jdv.16924.
Klaewsongkram J, Sukasem C, Thantiworasit P, Suthumchai N, Rerknimitr P, Tuchinda P, et al. Analysis of HLA-B allelic variation and IFN-γ ELISpot responses in patients with severe cutaneous adverse reactions associated with drugs. J Allergy Clin Immunol Pract. 2019;7(1):219–227.e4. https://doi.org/10.1016/j.jaip.2018.05.004.
Klaewsongkram J, Thantiworasit P, Suthumchai N, Rerknimitr P, Sukasem C, Tuchinda P, et al. In vitro test to confirm diagnosis of allopurinol-induced severe cutaneous adverse reactions. Br J Dermatol [Internet]. 2016 Nov [cited 2019 May 24];175(5):994–1002. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27106261.
•• Suthumchai N, Srinoulprasert Y, Thantiworasit P, Rerknimitr P, Tuchinda P, Chularojanamontri L, et al. The measurement of drug-induced interferon γ-releasing cells and lymphocyte proliferation in severe cutaneous adverse reactions. J Eur Acad Dermatol Venereol. 2018;32(6):992–8 This is a very interesting study showing good correlation between IFN-g ELISpot in the acute phase and LTT results in the recovery phase, demonstrating the usefulness of these in vitro tools to identify culprit drugs in SCARs.
Trubiano JA, Strautins K, Redwood AJ, Pavlos R, Konvinse KC, Aung AK, et al. The combined utility of ex vivo IFN-γ release enzyme-linked immunospot assay and in vivo skin testing in patients with antibiotic-associated severe cutaneous adverse reactions. J Allergy Clin Immunol Pract. 2018;6(4):1287–1296.e1. https://doi.org/10.1016/j.jaip.2017.09.004.
Konvinse KC, Trubiano JA, Pavlos R, James I, Shaffer CM, Bejan CA, et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol:1–10. https://doi.org/10.1016/j.jaci.2019.01.045.
Gualtieri B, Solimani F, Hertl M, Buhl T, Möbs C, Pfützner W. Interleukin 17 as a therapeutic target of acute generalized exanthematous pustulosis (AGEP). J Allergy Clin Immunol Pract. 2020;8(6):2081–2084.e2. https://doi.org/10.1016/j.jaip.2020.01.045.
Meier-Schiesser B, Feldmeyer L, Jankovic D, Mellett M, Satoh TK, Yerly D, et al. Culprit drugs induce specific IL-36 overexpression in acute generalized exanthematous pustulosis. J Invest Dermatol. 2019;139(4):848–58. https://doi.org/10.1016/j.jid.2018.10.023.
Beeler A, Zaccaria L, Kawabata T, Gerber BO, Pichler WJ. CD69 upregulation on T cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy [Internet]. 2008 Feb 13 [cited 2019 May 19];63(2):181–8. Available from: http://doi.wiley.com/10.1111/j.1398-9995.2007.01516.x.
Trubiano JA, Redwood A, Strautins K, Pavlos R, Woolnough E, Chang CC, et al. Drug-specific upregulation of CD137 on CD8+ T cells aids in the diagnosis of multiple antibiotic toxic epidermal necrolysis. J Allergy Clin Immunol Pract. 2017;5(3):823–6. https://doi.org/10.1016/j.jaip.2016.09.043.
•• Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J … [Internet]. 2013 [cited 2014 Mar 18];168(3):555–62. Available from: http://onlinelibrary.wiley.com/doi/10.1111/bjd.12125/full. Excellent multicentre study including the largest series of SCARs providing data on value and safety of patch tests in DRESS, SJS/TEN and AGEP.
Lin Y-T, Chang Y-C, Hui RC-Y, Yang C-H, Ho H-C, Hung S-I, et al. A patch testing and cross-sensitivity study of carbamazepine-induced severe cutaneous adverse drug reactions. J Eur Acad Dermatol Venereol [Internet]. 2013 Mar [cited 2019 May 24];27(3):356–64. Available from: http://doi.wiley.com/10.1111/j.1468-3083.2011.04418.x.
Fernando SL. Ertapenem-induced acute generalized exanthematous pustulosis with cross-reactivity to other beta-lactam antibiotics on patch testing. Ann Allergy Asthma Immunol. 2013;111(2):139–40. https://doi.org/10.1016/j.anai.2013.05.015.
Bommarito L, Zisa G, Delrosso G, Farinelli P, Galimberti M. A case of acute generalized exanthematous pustulosis due to amoxicillin-clavulanate with multiple positivity to beta-lactam patch testing. Eur Ann Allergy Clin Immunol. 2013;45(5):178–80.
• Santiago F, Gonçalo M, Vieira R, Coelho S, Figueiredo A. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis. 2010;62(1):47–53. https://doi.org/10.1111/j.1600-0536.2009.01659.x They evaluate the safety and usefulness of patch testing in a large series of DRESS.
• Wolkenstein P, Chosidow O, Fléchet ML, Robbiola O, Paul M, Dumé L, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis. 1996;35(4):234–6. First publication on the value of patch tests in SCARs.
Pinho A, Coutinho I, Gameiro A, Gouveia M, Gonçalo M. Patch testing—a valuable tool for investigating non-immediate cutaneous adverse drug reactions to antibiotics. J Eur Acad Dermatol Venereol [Internet]. 2017 Feb [cited 2019 May 26];31(2):280–7. Available from: http://doi.wiley.com/10.1111/jdv.13796.
Wakelin SH, James MP. Diltiazem-induced acute generalised exanthematous pustulosis. Clin Exp Dermatol. 1995;20(4):341–4.
Assier H, Ingen-Housz-Oro S, Zehou O, Hirsch G, Chosidow O, Wolkenstein P. Strong reactions to diltiazem patch tests: plea for a low concentration. Contact Dermatitis. 2020;83(3):224–5.
Serrao V, Caldas Lopes L, Campos Lopes JM, et al. Acute generalized exanthematous pustulosis associated with diltiazem. Acta Medica Port. 2008;21:99–102.
Smeets TJ, Jessurun N, Härmark L, Kardaun SH. Clindamycin-induced acute generalised exanthematous pustulosis: five cases and a review of the literature. Neth J Med. 2016;74(10):421–8.
Liccioli G, Marrani E, Giani T, Simonini G, Barni S, Mori F. The first pediatric case of acute generalized exanthematous pustulosis caused by hydroxychloroquine. Pharmacology. 2019;104(1-2):57–9. https://doi.org/10.1159/000500406.
Salman A, Yucelten D, Akin Cakici O, Kepenekli Kadayifci E. Acute generalized exanthematous pustulosis due to ceftriaxone: report of a pediatric case with recurrence after positive patch test. Pediatr Dermatol. 2019;36(4):514–6.
Zaouak A, Ben Salem F, Charfi O, Hammami H, Fenniche S. Acute generalized exanthematous pustulosis induced by terbinafine in a child confirmed by patch testing. Int J Dermatol. 2019;58(2):e42–3. https://doi.org/10.1111/ijd.14259.
Demitsu T, Kosuge A, Yamada T, Usui K, Katayama H, Yaoita H. Acute generalized exanthematous pustulosis induced by dexamethasone injection. Dermatology. 1996;193(1):56–8. https://doi.org/10.1159/000246204.
Padial MA, Alvarez-Ferreira J, Tapia B, et al. Acute generalized exanthematous pustulosis associated with pseudoephedrine. Br J Dermatol. 2004;150:139–42.
Komericki P, Grims R, Kränke B, Aberer W. Acute generalized exanthematous pustulosis from dalteparin. J Am Acad Dermatol. 2007;57(4):718–21.
Machet P, Marcé D, Ziyani Y, Dumont M, Cornillier H, Jonville-Bera AP, et al. Acute generalized exanthematous pustulosis induced by iomeprol with cross-reactivity to other iodinated contrast agents and mild reactions after rechallenge with iopromide and oral corticosteroid premedication. Contact Dermatitis. 2019;81(1):74–6.
Romano A, Di Fonso M, Pocobelli D, et al. Two cases of toxic epidermal necrolysis caused by delayed hypersensitivity to beta-lactam antibiotics. J Investig Allergol Clin Immunol. 1993;3:53–5.
Tagami H, Tatsuta K, Iwatski K, Yamada M. Delayed hypersensitivity in ampicillin-induced toxic epidermal necrolysis. Arch Dermatol. 1983;119:910–3.
Teo SL, Santosa A, Bigliardi PL. Stevens-Johnson syndrome/toxic epidermal necrolysis overlap induced by fexofenadine. J Investig Allergol Clin Immunol. 2017;27(3):191–3. https://doi.org/10.18176/jiaci.0158.
Sánchez I, García-Abujeta JL, Fernández L, et al. Stevens-Johnson syndrome from tetrazepam. Allergol Immunopathol (Madr). 1998;26(2):55–7.
Pietrantonio F, Moriconi L, Torino F, Romano A, Gargovich A. Unusual reaction to chlorambucil: a case report. Cancer Lett. 1990;54(3):109–11. https://doi.org/10.1016/0304-3835(90)90030-2.
Alonso R, Enrique E, Cistero A. Positive patch test to diclofenac in Stevens-Johnson syndrome. Contact Dermatitis. 2000;42(6):367.
Klein CE, Trautmann A, Zillikens D, Bröcker EB. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis. 1995;33(6):448–9. https://doi.org/10.1111/j.1600-0536.1995.tb02099.x.
Bensaid B, Rozieres A, Nosbaum A, Nicolas J-F, Berard F. Amikacin-induced drug reaction with eosinophilia and systemic symptoms syndrome: delayed skin test and ELISPOT assay results allow the identification of the culprit drug. J Allergy Clin Immunol. 2012;130(6):1413–4.
Caboni S, Gunera-Saad N, Ktiouet-Abassi S, Berard F, Nicolas JF. Esomeprazole-induced DRESS syndrome. Studies of cross-reactivity among proton-pump inhibitor drugs. Allergy. 2007;62(11):1342–3. https://doi.org/10.1111/j.1398-9995.2007.01428.x.
Shebe K, Ngwanya MR, Gantsho N, Lehloenya RJ. Severe recurrence of drug rash with eosinophilia and systemic symptoms syndrome secondary to rifampicin patch testing in a human immunodeficiency virus-infected man. Contact Dermatitis [Internet]. 2014 Feb [cited 2019 May 26];70(2):125–7. Available from: http://doi.wiley.com/10.1111/cod.12155.
Lehloenya RJ, Muloiwa R, Dlamini S, Gantsho N, Todd G, Dheda K. Lack of cross-toxicity between isoniazid and ethionamide in severe cutaneous adverse drug reactions: a series of 25 consecutive confirmed cases. J Antimicrob Chemother [Internet]. 2015 Sep [cited 2019 May 24];70(9):2648–51. Available from: https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dkv158.
Lehloenya RJ, Todd G, Wallace J, Ngwanya MR, Muloiwa R, Dheda K. Diagnostic patch testing following tuberculosis-associated cutaneous adverse drug reactions induces systemic reactions in HIV-infected persons. Br J Dermatol [Internet]. 2016 Jul [cited 2019 May 24];175(1):150–6. Available from: http://doi.wiley.com/10.1111/bjd.14492.
Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139(9):1181–3.
Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001;45(6):321–8.
Barbaud A. Drug patch testing in systemic cutaneous drug allergy. Toxicology [Internet]. 2005 Apr 15 [cited 2019 Jun 22];209(2):209–16. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0300483X04007267.
Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy Eur J Allergy Clin Immunol. 2002;57(1):45–51.
Romano A, Blanca M, Torres MJ, Bircher A, Aberer W, Brockow K, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy [Internet]. 2004 Nov [cited 2019 May 26];59(11):1153–60. Available from: http://doi.wiley.com/10.1111/j.1398-9995.2004.00678.x.
Romano A, Valluzzi RL, Caruso C, Maggioletti M, Gaeta F. Non-immediate cutaneous reactions to beta-lactams: approach to diagnosis. Curr Allergy Asthma Rep [Internet]. 2017 Apr 5 [cited 2019 May 26];17(4):23. Available from: http://link.springer.com/10.1007/s11882-017-0691-4.
• Soria A, Hamelin A, de Risi Pugliese T, Amsler E, Barbaud A. Are drug intradermal tests dangerous to explore cross-reactivity and co-sensitization in DRESS? Br J Dermatol. 2019;181(3):611–2. It is the largest study to assess the value and tolerance of drug intradermal tests in patients who had DRESS and negative patch tests. Interesting article.
Benamara-Levy M, Haccard F, Jonville Bera AP, Machet L. Acute generalized exanthematous pustulosis due to acetazolamide: negative on patch testing and confirmed by delayed-reading intradermal testing. Clin Exp Dermatol 2014;39(2):220–2.
Arruti N, Villarreal O, Bernedo N, Audicana MT, Velasco M, Uriel O, et al. Positive allergy study (intradermal, patch, and lymphocyte transformation tests) in a case of isoniazid-induced DRESS. J Investig Allergol Clin Immunol [Internet]. 2016 Apr 1 [cited 2019 May 19];26(2):119–20. Available from: http://www.jiaci.org/issues/vol26issue2/vol26issue02-10.htm.
Romano A, Viola M, Gaeta F, Rumi G, Maggioletti M. Patch testing in non-immediate drug eruptions. Allergy, Asthma Clin Immunol. 2008;4(2):66–74.
González-Ramos J, Noguera-Morel L, Tong HY, Ramírez E, Ruiz-Bravo E, Bellón T, et al. Two cases of overlap severe cutaneous adverse reactions to benznidazole treatment for asymptomatic Chagas disease in a nonendemic country. Br J Dermatol [Internet]. 2016 Sep [cited 2019 May 23];175(3):604–7. Available from: http://doi.wiley.com/10.1111/bjd.14451.
Jurado-Palomo J, Cabañas R, Prior N, Bobolea ID, Fiandor-Román a M, López-Serrano MC, et al. Use of the lymphocyte transformation test in the diagnosis of DRESS syndrome induced by ceftriaxone and piperacillin-tazobactam: two case reports. J Investig Allergol Clin Immunol [Internet]. 2010 Jan [cited 2019 May 23];20(5):433–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20945612.
• Phillips EJ, Bigliardi P, Bircher AJ, Broyles A, Chang YS, Chung WH, et al. Controversies in drug allergy: testing for delayed reactions. J Allergy Clin Immunol. 2019;143(1):66–73. https://doi.org/10.1016/j.jaci.2018.10.030. An international consensus document providing recommendations on controversies all over the world with regard to in vivo approaches to delayed immunologically mediated adverse drug reactions.
Huang LY, Liao WC, Chiou CC, Lou JP, Hu P, Ko FC. Fatal toxic epidermal necrolysis induced by carbamazepine treatment in a patient who previously had carbamazepine-induced Stevens-Johnson syndrome. J Formos Med Assoc. 2007;106(12):1032–7. https://doi.org/10.1016/S0929-6646(08)60079-0.
Schmidt D, Kluge W. Fatal toxic epidermal necrolysis following reexposure to phenytoin: a case report. Epilepsia. 1983;24(4):440–3. https://doi.org/10.1111/j.1528-1157.1983.tb04914.x.
Halevi A, Ben-Amitai D, Garty BZ. Toxic epidermal necrolysis associated with acetaminophen ingestion. Ann Pharmacother. 2000;34(1):32–4. https://doi.org/10.1345/aph.19064.
Park JJ, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. A case of hydroxychloroquine induced acute generalized exanthematous pustulosis confirmed by accidental oral provocation. Ann Dermatol. 2010;22(1):102–5. https://doi.org/10.5021/ad.2010.22.1.102.
Watts TJ, Li PH, Haque R. DRESS Syndrome due to benzylpenicillin with cross-reactivity to amoxicillin. J Allergy Clin Immunol Pract [Internet]. 2018 Sep [cited 2019 May 26];6(5):1766–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213219818300382.
Prados-Castaño M, Piñero-Saavedra M, Leguísamo-Milla S, Ortega-Camarero M, Vega-Rioja A. DRESS syndrome induced by meropenem. Allergol Immunopathol (Madr) [Internet]. 2015 Mar [cited 2019 May 26];43(2):233–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24731770.
Trubiano JA, Chua KYL, Holmes NE, Douglas AP, Mouhtouris E, Goh M, et al. Safety of cephalosporins in penicillin class severe delayed hypersensitivity reactions. J Allergy Clin Immunol Pract [Internet]. 2019 Oct [cited 2019 Nov 24]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213219819308943.
Pasricha JS, Khaitan BK, Shantharaman R, Mital A, Girdhar M. Toxic epidermal necrolysis. Int J Dermatol. 1996;35(7):523–7. https://doi.org/10.1111/j.1365-4362.1996.tb01674.x.
Katoh N, Kagawa K, Yasuno H. Piroxicam induced Stevens-Johnson syndrome. J Dermatol. 1995;22(9):677–80.
Palmero D, Castagnino J, Musella RM, Mosca C, González Montaner P, de Casado GC. Difficult clinical management of anti-tuberculosis DRESS syndrome. Int J Tuberc Lung Dis [Internet]. 2013 Jan 1 [cited 2019 Jun 22];17(1):76–8. Available from: http://openurl.ingenta.com/content/xref?genre=article&issn=1027-3719&volume=17&issue=1&spage=76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rosario Cabañas, Elena Ramirez, and Teresa Bellon declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Drug Allergy
Rights and permissions
About this article
Cite this article
Cabañas, R., Ramírez, E. & Bellón, T. Identifying the Culprit Drug in Severe Cutaneous Adverse Reactions (SCARs). Curr Treat Options Allergy 8, 194–209 (2021). https://doi.org/10.1007/s40521-021-00291-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-021-00291-1