Abstract
Background
Non-invasive brain stimulation (NIBS) is a burgeoning approach with the potential to significantly enhance cognition and functional abilities in individuals who have undergone a stroke. However, the current evidence lacks robust comparisons and rankings of various NIBS methods concerning the specific stimulation sites and parameters used. To address this knowledge gap, this systematic review and meta-analysis seek to offer conclusive evidence on the efficacy and safety of NIBS in treating post-stroke cognitive impairment.
Methods
A systematic review of randomized control trials (RCT) was performed using Bayesian network meta-analysis. We searched RCT in the following databases until June 2022: Cochrane Central Register of Controlled Trials (CENTRAL), PUBMED, and EMBASE. We compared any active NIBS to control in terms of improving cognition function and activities of daily living (ADL) capacity following stroke.
Results
After reviewing 1577 retrieved citations, a total of 26 RCTs were included. High-frequency (HF)-repetitive transcranial magnetic stimulation (rTMS) (mean difference 2.25 [95% credible interval 0.77, 3.66]) was identified as a recommended approach for alleviating the global severity of cognition. Dual-rTMS (27.61 [25.66, 29.57]) emerged as a favorable technique for enhancing ADL function. In terms of stimulation targets, the dorsolateral prefrontal cortex exhibited a higher ranking in relation to the global severity of cognition.
Conclusions
Among various NIBS techniques, HF-rTMS stands out as the most promising intervention for enhancing cognitive function. Meanwhile, Dual-rTMS is highly recommended for improving ADL capacity.
Similar content being viewed by others
Introduction
Stroke is a prominent contributor to morbidity and mortality on a global scale, imposing substantial challenges for patients, caregivers, and healthcare systems [1, 2]. As a prevalent residual complication, poststroke cognitive impairment (PSCI) is presented in nearly 70% of stroke survivors and has been implicated with unfavorable long-term outcomes [3].
Depending on the type of stroke, characteristics of the population, and time point and thresholds of assessments, the underlying cognitive profile of PSCI can be varied. Still, it is widely acknowledged that PSCI can exert deficits not only specific to stroke lesion sites but also in other cognitive networks associated with higher executional and attentional function [4], hampering the quality of life, affecting activities of daily living (ADL), and potentially elevating the risk of recurrent stroke [5]. As such, the restoration of post-stroke cognitive function has gradually received recognition equivalent to motor function [6, 7].
In the last two decades, non-invasive brain stimulation (NIBS) has emerged as a promising strategy to preserve cognitive function after stroke [8,9,10]. Based on the interhemispheric inhibition model, NIBS techniques collectively employ electrical or magnetic energy to achieve the balance of excitability between two hemispheres [11, 12]. The conventional NIBS techniques for PSCI include repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS).
rTMS operates by positioning a coil on the scalp to deliver brief bursts of electricity, generating a pulsating magnetic field. rTMS is categorized into high-frequency rTMS (excitatory, 3–20 Hz) and low-frequency rTMS (inhibitory, ≤ 1Hz) based on various frequency parameters [13]. Novel forms of rTMS like Theta-burst stimulation (TBS) are divided into intermittent TBS (iTBS) and continuous TBS (cTBS) [14, 15]. tDCS works by applying a weak and continuous direct current to the cerebral cortex, possessing the capability to either augment or diminish cortical excitability [16, 17]. Low-intensity direct current can alter the excitability of the cerebral cortex. Excitatory anodal tDCS and inhibitory cathodal tDCS are common neurorehabilitation modes clinically.
Although the effects of NIBS have been critically evaluated in previous meta-analyses, the incomplete findings, particularly on efficacy comparison and specific stimulation sites, have hindered the integration of NIBS into standard clinical practice [18,19,20]. Considering the aforementioned challenges, our objective is to conduct a thorough comparison and ranking of the effectiveness of different NIBS modalities in enhancing both post-stroke cognitive function and ADL function. Additionally, we aim to assess the domain-specific effects on various cognitive domains through the use of network meta-analysis (NMA). This approach will provide the most comprehensive and robust evidence currently available [21].
Methods
This study followed the PRISMA guideline [22]. Furthermore, it was prospectively registered in the PROSPERO database of systematic reviews under the registration number CRD42022342903 [23].
Search strategy
From January 2012 to June 2022, a systematic search was performed in three electronic databases, including PubMed, Embase, and the Cochrane Library. There were no language or other restrictions. The comprehensive strategy, including search terms tailored for each database, is available in Supplementary Appendix 2.
Inclusion and exclusion criteria
Two independent investigators (MY and JL) carried out the selection of records based on screening criteria that involved filtering through titles, abstracts, and full texts. If any discrepancies arose, a third reviewer (YL) was consulted for resolution.
The inclusion criteria for the studies were as follows: (1) participants: individuals diagnosed with a stroke, confirmed through standardized scales and neuroimaging; (2) intervention: Non-invasive brain stimulation (NIBS) modes, including rTMS, tDCS, and other variants; (3) comparison: placebo therapy or sham stimulation. In cases where combined interventions were utilized, the control group received the same non-invasive brain stimulation component of the intervention (e.g., brain stimulation plus conventional rehabilitation therapy vs. sham plus conventional rehabilitation therapy); (4) outcome: Changes in scale values or performance on cognitive and ADL tasks after therapy, including Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Rivermead Behavioural Memory Test (RBMT), Trail Making Test (TMT), Line Bisection Test (LBT), Star Cancellation Test(SCT), Catherine Bergego Scale(CBS), Modified Barthel Index (MBI), Barthel Index (BI), Functional Independence Measure (FIM) and National Institutes of Health Stroke Scale (NIHSS) [24,25,26,27,28,29,30,31], with studies reporting sufficient information to compute common effect size statistics (i.e., mean and standard deviations [SD], exact F-, p-, t-, or z-values); (5) study design: Randomized controlled trials (RCTs) involving adult participants (≥ 18 years). Studies were not considered for inclusion if they met any of the following exclusion criteria: (1) publication in the form of abstracts; (2) implementation of interventions that were irrelevant or imbalanced across different groups (e.g., invasive interventions); (3) failure to report pre-post changes in cognitive performance or inability to calculate these changes based on the available data (e.g., inconsistent results not following the consolidated standards using computerized measurement systems); and (4) study types that could not provide reliable data according to GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology [32], the initial quality assessment corresponds to the study design, i.e., “high” for experimental studies (eg, randomized clinical trials [RCTs]) and “low” for observational studies (e.g., case reports and cohort studies), including case reports and cohort studies.
Data extraction
Two reviewers (MY and JL) extracted data from eligible studies independently and assessed by another experienced investigator (YL). A pre-specified form showed demographic features, study characteristics, clinical information and stimulation parameters. The outcome data of cognition level and activities of daily living function were collected from the main text, tables and supplementary materials. The results of outcome assessment values in the figures were carried out using Engauge Digitizer (version 12.1).
Quality assessment
Two independent reviewers (JS and XW) assessed the quality of the included studies using the Cochrane collaboration’s tool for assessing the risk of bias in randomized trials (RoB) in Review Manager (version 5.4.1). RoB was based on the Cochrane Handbook recommendations and explored sources of bias based on seven dimensions, namely the risk of bias in six domains: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias [33]. (as shown in Supplementary Figs. 3 and 4).
Primary and secondary outcomes
Pre-post changes in the global severity of cognitive impairment were considered as the primary outcomes. The primary outcomes of global cognition severity were quantified using MMSE and MoCA [24]. The assessment of subdomain scales, such as executive function, memory, and perception, served as secondary outcomes in the study. These scales included RBMT [25], TMT [26], LBT, SCT, CBS [27] and Motor-Free Visual Perception Test (MVPT).
Secondary outcomes in this study also included the MBI [28], BI [29], FIM [30], and NIHSS) [31]. These scales were used to assess the overall severity of daily living abilities and stroke. Further details about these scales can be found in Supplementary Appendix 3. Adverse events and dropouts are also summarized in Supplementary Appendix 4.
Statistical analysis
An NMA based on a Bayesian random-effects framework was performed using R (version 4.1.3, gemtc package (1.0–1)). In this study, a Markov chain Monte Carlo method was employed to combine the direct and indirect comparisons of interventions, with the number of chains set at four. Gibbs sampling [34] was according to 20,000 iterations by removing 5,000 iterations in the burn-in phase. The effect size of therapeutic efficacy for continuous variables, as measured by scales, was evaluated using the pooled mean differences (MDs) of pre-post cognitive changes along with their corresponding 95% credible intervals (CrIs). Given that some studies did not directly report change values, we derived the standard deviations (SDs) of pre-post changes by utilizing the original datasets acquired from online supplements or obtained through requests made to the respective authors. In cases where the aforementioned methodologies were not available, we employed a simple imputation approach to estimate the SD values, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions [35]. To rank the therapy efficacies of each outcome, the surface under the cumulative ranking curve (SUCRA) was computed, with a higher SUCRA value, approaching 100%, suggesting a higher likelihood of an intervention ranking among the top positions [36]. The level of statistical significance was set to p < 0.05.
Inconsistency of model
To assess inconsistency, the 'mtc.nodesplit' method was employed, and a significance level of p < 0.05 was utilized to determine whether the inconsistency was statistically significant.
Results
Study selection
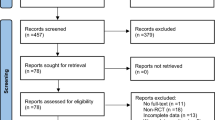
The study selection process is presented in Fig. 1. A total of 1577 records were initially obtained from databases. After removing 416 duplicate records, 1161 records remained. Following the screening of titles and abstracts to exclude irrelevant data, 292 full-text reports were selected for further assessment of eligibility. Trials conducted by a specific research team were included as long as they involved different populations or interventions within the study cohort. A total of 26 RCT reports were included in the meta-analysis.
Study characteristics
The detailed characteristics of individual studies are listed in Table 1. This meta-analysis included 1062 patients with PSCI. The average proportion of females in the included studies was 42.5%, and the average age of the participants was 58.91 ± 12.14 years. Among the enrolled patients, a small subset (25%, 5 out of 20) were in the chronic phase of stroke recovery (more than 6 months), while 70% (14 out of 20) were in the subacute phase (7 days to 6 months). By the way, only one trial [37] was in the acute phase and one trial [48] was categorized as both subacute and chronic post-stroke. Five trials [42, 46, 53, 56, 57] did not provide information about the duration of the disease course. Two clinical trials [38, 53] did not report participant ages.
Concerning the details of the therapy interventions, each therapy lasted a mean of 15.8 ± 5.7 (6–30) sessions, and rTMS demonstrated to be the most frequent form of NIBS for addressing cognitive and functional deficits following stroke. Out of the total number of trials, 18 (69.2%) focused on rTMS, encompassing 13 conventional rTMS trials, 4 TBS trials, and 1 trial [49] combining both types of stimulation. A significant proportion of the trials focused on measuring the primary outcomes related to the global severity of post-stroke cognitive impairments. Various cognition scales, such as MMSE, MoCA, TMT, RBMT, MVPT, LBT, SCT, and CBS, were employed for outcome assessments. Additionally, outcome measurements also included global severity assessments of ADL and stroke using scales such as BI, MBI, FIM, and NIHSS. Detailed information can be found in Supplementary Table 1.
Risk of bias
The risk of bias in the included studies is summarized in Supplementary Fig. 3. Across the seven domains of the PRISMA RoB tool, two domains were identified to have a high risk of bias (as shown in Supplementary Fig. 4).
Network geometry of intervention
Figure 2 presents the geometry of the treatment networks across each cognition domain in the short-term assessment of the primary and secondary outcomes. As shown in Fig. 2A, the trials reporting cognition function measured by MMSE contained six interventions and eight pairs of direct comparisons. Another scale called MoCA contained four interventions and three edges. Furthermore, Fig. 2A illustrates the network geometry of ADL, which consists of five nodes and seven edges for BI, five nodes and six edges for MBI, and six nodes and five edges for FIM. The global severity of the stroke, as measured by NIHSS, is represented by a network with six nodes and nine edges. Across the subdomains (see Supplementary Fig. 1A), the visual perception network showed by LBT and SCT had the highest number of nodes (seven interventions) and edges (eleven comparisons). Other subdomain network geometry included TMT (four nodes, four edges) for executive function, RBMT (three nodes, two edges) for memory function, and MVPT (five nodes, six edges) and CBS for perception function (six nodes, seven edges).
Network geometry of interventions across cognition and ADL function in the short-term assessment. A Gross network plots of NIBS modalities for global severity of cognition and ADL function as defined by stimulation parameters; B Refined network plots of NIBS subtypes for global severity of cognition and ADL function by targeted stimulation location. Each node in the diagram represents an intervention, with its size reflecting the sample size of patients involved. The edges connecting the nodes represent direct comparisons between interventions, and their width is proportional to the number of trials conducted for each specific comparison. HF- high frequency, LF- low frequency, cTBS continuous theta burst stimulation, a- anodal, c- cathodal, DLPFC dorsolateral prefrontal cortex, STG superior temporal gyrus, FTP fronto-temporal region, PPC posterior parietal cortex, M1 primary motor cortex, NIBS non-invasive brain stimulation, ADL activities of daily living, rTMS repetitive transcranial magnetic stimulation, tDCS transcranial direct current stimulation
Refined networks subdivided by targeted brain regions are presented in Fig. 2B and Supplementary Fig. 1B.
Efficacy across cognition, living function and stimulation regions according to pooled MDs
Regarding the measurement of cognition and living function, the present review included two interventions (rTMS and tDCS). There were 26 studies included in our network meta-analysis enrolling 1062 stroke patients. Rankings of the effects of NIBS modalities measured with SUCRAs are shown in Fig. 3.
Rankings of the effects of different NIBS modalities on cognition recovery measured with SUCRAs (surface under the cumulative ranking curve). SUCRAs (values range from 0 to 1) for each cognition domain and ADL function are shown in radar graphs as defined by stimulation parameters (A) and by targeted stimulation location (B). The SUCRA value represents the likelihood of an intervention being ranked as the highest. Colored lines indicate the effect sizes of interventions compared to placebo. HF- high frequency, LF- low frequency, cTBS continuous theta burst stimulation, a- anodal, c- cathodal, DLPFC dorsolateral prefrontal cortex, STG superior temporal gyrus, FTP fronto-temporal region, PPC posterior parietal cortex, M1 primary motor cortex, NIBS non-invasive brain stimulation, ADL activities of daily living, rTMS repetitive transcranial magnetic stimulation, MD mean difference, tDCS transcranial direct current stimulation
The global severity of cognition
Combining the forest plots and the SUCRA value, it can be suggested that HF-rTMS (MD 2.25 [95% CrI 0.77, 3.66]) has shown to be the most effective therapy in improving global cognitive severity. None of the interventions demonstrated significant efficacy in improving the outcome of MoCA. HF-rTMS (with a mean difference of 4.14 [− 0.28, 9.22]) was ranked among the other treatment modalities (refer to Fig. 4A and Table 2).
Forest plots of network meta-analyses compared with placebo across various cognition domains and ADL function, pooling the effects of NIBS modalities (number of trials ≥ 2). A Forest plots of NIBS modalities for global severity of cognition and ADL function as defined by stimulation parameters; B forest plots of refined NIBS subtypes for global severity of cognition and ADL function by targeted stimulation location. HF- high frequency, LF- low frequency, cTBS continuous theta burst stimulation, a- anodal, c- cathodal, DLPFC dorsolateral prefrontal cortex, STG superior temporal gyrus, FTP fronto-temporal region, M1 primary motor cortex, NIBS non-invasive brain stimulation, ADL activities of daily living, rTMS repetitive transcranial magnetic stimulation, MD mean difference, tDCS transcranial direct current stimulation
Regarding targeted brain regions, it can be considered that HF-rTMS-DLPFC (2.51 [0.64, 4.46]) is the best among these interventions in improving the global severity of cognition from MMSE. Again, none of the interventions also show apparent efficacy in enhancing global cognitive function. However, like the results from MMSE, based on the SUCRA value, HF-rTMS-DLPFC (4.13 [− 0.24, 9.25]) was also ranked among these modes in the measurement of MoCA (see Fig. 4B and Supplementary Table 2).
ADL function
Considering the BI index, Dual-rTMS had a superior effect size (27.61 [25.66, 29.57]), and significant advantages were also observed with LF-rTMS (12.80 [10.78, 14.84]) and HF-rTMS (8.60 [6.66, 10.56]). Same results come from the MBI index, HF-rTMS (9.31 [6.28, 12.38]) and LF-rTMS (10.70 [9.00, 12.38]) had priority among these modes. LF-rTMS (2.74 [1.12, 4.37]), HF-rTMS (2.09 [1.37, 2.81]), and a-tDCS (1.62 [1.31, 1.93]) had the same advantages on the stroke recovery of the NIHSS scale (refer to Fig. 4A and Table 2).
In terms of the targeted brain regions, among the specific modes of NIBS, Dual-rTMS-M1 exhibited the most favorable effect size (27.61 [25.66, 29.57]) in improving BI. HF-rTMS-DLPFC (8.07 [4.74, 11.39]), LF-rTMS-DLPFC (9.65 [5.01, 14.15]), LF-rTMS-PPC (mean difference 4.06 [95% CrI 0.13, 8.02]), and HF-rTMS-PPC (13.45 [6.18, 20.71]) all exhibited advantages in improving MBI. Furthermore, Dual-rTMS-M1 (4.95 [3.20, 6.70]), LF-rTMS-M1 (2.79 [0.83, 4.75]), HF-rTMS-M1 (2.15 [0.53, 3.79]), HF-rTMS-DLPFC (2.08 [1.29, 2.87]), and a-tDCS-STG (1.62 [1.31, 1.93]) all showed improvements in the rehabilitation process of stroke according to NIHSS (see Fig. 4B and Supplementary Table 2).
Subdomains of cognition
LF-rTMS (3.89 [2.05, 5.95]) had impressive privileges in the MVPT scale. HF-rTMS (19.12 [3.52, 34.94]), LF-rTMS (13.03 [1.30, 24.78]) had considerable improvement among these NIBS modes in the results of the LBT scale. Three modes, including HF-rTMS (1.85 [0.94, 2.76]), a-tDCS (1.77 [1.54, 2.00]), and c-tDCS (1.17 [0.92, 1.42]), had the same positive influence in the executive function of TMT scale. Only LF-rTMS (2.81 [1.47, 4.14]) offered benefits in the results of the RBMT scale (see Supplementary Fig. 2A).
Regarding targeted brain regions, LF-rTMS-PPC (3.92 [1.83, 6.21]) had a moderate privilege in the MVPT scale. HF-rTMS-PPC (19.12 [3.34, 35.24]), LF-rTMS-PPC (13.08 [1.20, 25.18]) had considerable improvement among these NIBS modes in the results of the LBT scale. Three modes, including HF-rTMS- DLPFC (1.86 [0.94, 2.77]), a-tDCS-STG (1.77 [1.53, 2.00]), and c-tDCS-STG (1.17 [0.92, 1.42]), had the same positive influence in the executive function of TMT scale. In addition, LF-rTMS-DLPFC (2.81 [1.48, 4.14]) offered evident benefits in the results of the RBMT scale (see Supplementary Fig. 2B).
Adverse events and dropouts
Participants in both rTMS and tDCS trials reported experiencing pricking sensations and tingling. Furthermore, a small percentage of trials (11%, 3 out of 26) [37, 40, 48] documented the occurrence of mild symptoms, such as temporary headaches and dizziness in the rTMS sessions. No severe adverse events were documented in any of the trials included in the analysis. Overall, both rTMS and tDCS are considered relatively safe, as no patients withdrew from the trials due to serious adverse effects.
Evaluation of the inconsistency
Test results for outcomes that met the test criteria were shown by stimulation type and by targeted stimulation location (See Supplementary Fig. 5).
Discussion
The present NMA assessed the efficacy of seven NIBS modalities across 1062 patients with cognition deficits at various levels. Most NIBS research on these symptoms finds positive effects across several cognitive subdomains. HF-rTMS ranks the highest in improving the global severity of cognition. Considering the subdomains, LF-rTMS is ranked higher in improving memory and unilateral spatial neglect (USN). a-tDCS has a positive effect on the global severity of stroke, USN and executive function. Taken together, Dual-rTMS is recommended for enhancing ADL function. Refined NMA based on locations find that DLPFC stimulation has a clear advantage, especially regarding global severity. Also, PPC is the best choice for USN. Moreover, M1 stimulation exhibits a significant influence on the BI and NIHSS scales. Additionally, the STG site plays a vital role in executive function.
Domain-specific rankings across cognition aspects and ADL
We focus on examining the effectiveness of various treatments throughout the stroke recovery process. The enhancement of cognition and ADL capacity could potentially have a broad positive impact on stroke patients [41]. Therefore, we rank the efficacy of these functions in terms of stroke.
We find that the overall effects tend to be more favorable for bilateral stimulation, particularly in relation to ADL function. According to a study by Qingmei Chen et al. [37], the application of HF-rTMS and LF-rTMS on both the affected and unaffected hemispheres provides additional therapeutic benefits for functional recovery. Other studies have shown alignment, where Dual-rTMS exerts significant protective effects on the risk of worsening cognitive decline [61]. We speculate that applying Dual-rTMS can alleviate the interhemispheric inhibition interaction and promote recruiting collaborative potential between two hemispheres motor cortices across varying injury extents when comparing bilateral stimulation to unilateral stimulation. Regardless, more studies are required to explore the unidentified neurophysiological mechanisms of synergistic advantages in Dual-rTMS.
The NMA suggests a favorable effect of a-tDCS in the global severity of a stroke, USN and executive function. The advantageous mechanism of anodal transcranial direct current stimulation (a-tDCS), similar to HF-rTMS mentioned previously. In the NMA of Bernhard Elsner et al. [62], c-tDCS is the most promising treatment option to improve ADL capacity and arm function in patients with stroke, which is contradictory to our results partly. However, this NMA included a large proportion of c-tDCS studies, which may affect the conclusion of the superiority of c-tDCS with the advantages of publication bias. In general, there is controversy surrounding the effectiveness of different types of tDCS in stroke recovery.
Concerning stimulation modes, rTMS works at more localized areas under the coil, whereas tDCS stimulates less focal and more broadly brain regions. Meanwhile, tDCS presents a more cost-effective and convenient option compared to rTMS, making it a viable alternative for clinical intervention.
Selection of targeted brain regions contributes to rehabilitation
There is insufficient evidence to precisely address the underlying mechanism of rTMS and tDCS in neurorehabilitation among PSCI patients. Various factors, such as distinct pathophysiological mechanisms, heterogeneity, may influence the outcomes of stimulation types. Therefore, brain region selection is crucial for increasing functional connectivity within the brain network.
Dorsolateral prefrontal cortex (DLPFC) is the frequently targeted cortical site for cognitive recovery using NIBS techniques. Additionally, the DLPFC has been associated with executive functions, as per a recent organizing principle that distinguishes cognitive processes from affective/reward-related processes [63]. A previous study [64] showed that DLPFC stimulation can amplify DMN node deactivations and enhance high cognitive demand processing. It can maximize therapeutic efficacy through the integration of HF-rTMS stimulation for unleashing the potential of neuronal recovery through enhanced excitability of ipsilesional hemisphere. Thus, DLPFC is favored selection for cognition recovery.
Further, stimulating the motor cortex has been found to improve ADL function. Augmenting motor cortical excitability in the hemisphere affected by stroke is a crucial prerequisite for neural plasticity. This allows the remaining neurons to reorganize and adapt in response to treatment feedback. As a result, motor cortex (M1) stimulation has shown additional benefits across multiple domains, including enhanced ADL capacity and alleviation of cognitive dysfunction [65].
The superior temporal gyrus (STG) is located in the upper part of the temporal lobe and plays a role in both verbal and non-verbal communication. The right anterior STG is involved in processing object- and space-related information. The left posterior STG is responsible for language processing, auditory short-term memory, and the perception and production of speech [66, 67]. Therefore, this region may contribute to the recovery of executive function.
Anatomical-clinical data suggests that posterior parietal damage is the most common relevant anatomical area for USN. Meanwhile, USN is associated with decreased arousal network activity and an imbalance of cortico-subcortical hemispheric connectivity [68]. According to Maurizio Corbetta et al. [69], the phenomenon of neglect is better understood as a result of impairments in distributed cortical networks responsible for attention control, rather than being solely attributed to structural damage in specific brain regions. Overall, PPC stimulation still has significant effects in improving USN.
c-tDCS and other brain regions not mentioned did not show prominent priority in cognition and living function, maybe more extensive clinical trials with larger sample sizes are required to achieve solid evidence.
Taking into account the aforementioned factors, the combination of an appropriate brain region with rTMS has the potential to yield more favorable therapeutic outcomes. In addition, the restoration of entire brain networks plays a crucial role in the rehabilitation journey of stroke patients.
Strengths and limitations
To the best of our knowledge, this is the first NMA to rank the efficacy of NIBS modes and targeted brains for function in cognition and daily living of patients with stroke. Additionally, evidence-based clinical decision-making can be enhanced by utilizing NMA, which combines direct and indirect comparisons of trials. This approach provides ranked results that indicate the relative efficacy of each intervention type, aiding in informed decision-making. Moreover, specific studies that primarily focus on enhancing motor function have been deemed valuable additions to NMA in terms of statistical integrity, as they also offer potential benefits for cognition and ADL. Overall, the primary goal of this NMA is to offer improved rehabilitation strategies for patients and provide clinicians with enhanced decision support.
Notwithstanding, our study does have a few limitations. First, part of NIBS modes was not included in the current NMA, which may have an impact on the completeness of the findings. However, the inclusion has covered common clinical treatments and explains and guides current clinical applications. Second, the assessment of outcomes in our study involved the utilization of different cognition scales, which introduced subjectivity and potentially increased heterogeneity. To mitigate this bias, we specifically selected RCTs that incorporated blinding designs. Additionally, we included as many relevant scales as possible to ensure comprehensive results. However, given the diversity of assessments, it was not feasible to include all interventions in the partial ranking. Therefore, the results of our study should only be applied to the interventions included in the analysis.
Conclusions
HF-rTMS is recognized as the superior NIBS intervention for ameliorating overall cognitive impairment. In contrast, Dual-rTMS has exhibited greater effectiveness in improving ADL functioning, while LF-rTMS has demonstrated advantages in enhancing memory and alleviating USN. For enhancing global cognition, DLPFC is highly recommended, whereas the motor cortex (M1) can also be beneficial for improving ADL function. Additionally, PPC is a recommended option for alleviating USN.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Feigin VL, Stark BA, Johnson CO et al (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20:795–820
Donnan GA et al (2008) Stroke. Lancet 371:1612–1623
Oksala NKJ et al (2009) Cognitive impairment predicts poststroke death in long-term follow-up. J Neurol Neurosurg Psychiatry 80:1230–1235
Jokinen H et al (2015) Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol 22:1288–1294
Kwon HS et al (2020) Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study. J Neurol 267:688–693
Pantoni L, Salvadori E (2021) Location of infarcts and post-stroke cognitive impairment. Lancet Neurol 20:413–414
Cicerone KD et al (2011) Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil 92:519–530
Park S-H et al (2013) A double-blind, sham-controlled, pilot study to assess the effects of the concomitant use of transcranial direct current stimulation with the computer assisted cognitive rehabilitation to the prefrontal cortex on cognitive functions in patients with stroke. J Korean Neurosurg Soc 54:484–488
Cazzoli D et al (2012) Theta burst stimulation reduces disability during the activities of daily living in spatial neglect. Brain J Neurol 135:3426–3439
Li H et al (2021) Repetitive transcranial magnetic stimulation (rTMS) modulates thyroid hormones level and cognition in the recovery stage of stroke patients with cognitive dysfunction. Med Sci Monitor Int Med J Exp Clin Res 27:e931914
Du J et al (2019) Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: Evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. NeuroImage Clin 21:101620
Elsner B et al (2020) Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database System Rev 11:CD009645
Lefaucheur J-P et al (2020) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol Off J Int Feder Clin Neurophysiol 131:474–528
Jung J, Lambon Ralph MA (2016) Mapping the dynamic network interactions underpinning cognition: a cTBS-fMRI study of the flexible adaptive neural system for semantics. Cerebral Cortex (New York, N.Y.: 1991) 26: 3580–3590
Pabst A et al (2022) A systematic review and meta-analysis of the efficacy of intermittent theta burst stimulation (iTBS) on cognitive enhancement. Neurosci Biobehav Rev 135:104587
Nitsche MA et al (2003) Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol Off J Int Feder Clin Neurophysiol 114:600–604
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57:1899–1901
Begemann MJ et al (2020) Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med 50:2465–2486
Liu M et al (2021) The role of repetitive transcranial magnetic stimulation in the treatment of cognitive impairment in stroke patients: A systematic review and meta-analysis. Sci Prog 104:368504211004266
Hara T, Shanmugalingam A, McIntyre A et al (2021) The effect of non-invasive brain stimulation (NIBS) on attention and memory function in stroke rehabilitation patients: a systematic review and meta-analysis. Diagnostics (Basel) 11:227. https://doi.org/10.3390/diagnostics11020227
Rouse B, Chaimani A, Li T (2017) Network meta-analysis: an introduction for clinicians. Intern Emerg Med 12:103–111
Hutton B et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162:777–784
Mengyu Yan JS (2022) Comparison of the efficacy of different non-invasive brain stimulation on cognitive function after stroke: a systematic review and meta-analysis. PROSPERO 2022 CRD42022342903. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=342903
Khaw J, Subramaniam P, Abd Aziz NA et al (2021) Current update on the clinical utility of MMSE and MoCA for stroke patients in Asia: a systematic review. Int J Environ Res Pub Health 18:8962. https://doi.org/10.3390/ijerph18178962
Yassuda MS et al (2010) Psychometric characteristics of the Rivermead Behavioural Memory Test (RBMT) as an early detection instrument for dementia and mild cognitive impairment in Brazil. Int Psychogeriatr 22:1003–1011
Llinàs-Reglà J et al (2017) The trail making test. Assessment 24:183–196
Luukkainen-Markkula R et al (2011) Comparison of the behavioural inattention test and the catherine Bergego Scale in assessment of hemispatial neglect. Neuropsychol Rehabil 21:103–116
Ohura T et al (2017) Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol 17:131
Duffy L et al (2013) Reliability (inter-rater agreement) of the Barthel Index for assessment of stroke survivors: systematic review and meta-analysis. Stroke 44:462–468
Brown AW et al (2015) Measure of functional independence dominates discharge outcome prediction after inpatient rehabilitation for stroke. Stroke 46:1038–1044
Kwah LK, Diong J (2014) National Institutes of Health Stroke Scale (NIHSS). J Physiother 60:61
Guyatt GH et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research ed) 336:924–926
Higgins JPT et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Xu C et al (2018) Software and package applicating for network meta-analysis: a usage-based comparative study. J Evid Based Med 11:176–183
Cumpston M et al (2019) Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database System Rev 10:ED000142
Salanti G, Ades AE, Ioannidis JPA (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64:163–171
Chen Q et al (2021) Effects of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation on motor recovery in patients following acute cerebral infarction. NeuroRehabilitation 48:83–96
Yu F, He R (2021) The effect of fluoxetine combined with repetitive transcranial magnetic stimulation on the psychological emotions and cognitive and neurological functions of acute post-stroke depression patients. Am J Transl Res 13:11883–11889
Yin M et al (2020) Effects of rTMS treatment on cognitive impairment and resting-state brain activity in stroke patients: a randomized clinical trial. Front Neural Circuits 14:563777
Li Y et al (2020) Cerebral functional manipulation of repetitive transcranial magnetic stimulation in cognitive impairment patients after stroke: an fMRI study. Front Neurol 11:977
Liu Y et al (2020) Effects of transcranial magnetic stimulation on the performance of the activities of daily living and attention function after stroke: a randomized controlled trial. Clin Rehabil 34:1465–1473
Vatanparasti S et al (2019) The effect of continuous theta-burst transcranial magnetic stimulation combined with prism adaptation on the neglect recovery in stroke patients. J Stroke Cerebrovasc Dis 28:104296
Nyffeler T et al (2019) Theta burst stimulation in neglect after stroke: functional outcome and response variability origins. Brain 142:992–1008
Kim SB et al (2018) Effect of combined therapy of robot and low-frequency repetitive transcranial magnetic stimulation on hemispatial neglect in stroke patients. Ann Rehabil Med 42:788–797
Askin A, Tosun A, Demirdal US (2017) Effects of low-frequency repetitive transcranial magnetic stimulation on upper extremity motor recovery and functional outcomes in chronic stroke patients: a randomized controlled trial. Somatosens Mot Res 34:102–107
Kim K-U, Kim S-H, An T-G (2017) The effects of repetitive transcranial magnetic stimulation (rTMS) on depression, visual perception, and activities of daily living in stroke patients. J Phys Ther Sci 29:1036–1039
Hosomi K et al (2016) Daily repetitive transcranial magnetic stimulation for poststroke upper limb paresis in the subacute period. J Stroke Cerebrovasc Dis 25:1655–1664
Lu H et al (2015) Impact of repetitive transcranial magnetic stimulation on post-stroke dysmnesia and the role of BDNF Val66Met SNP. Med Sci Monit 21:761–768
Yang W et al (2015) Comparison of different stimulation parameters of repetitive transcranial magnetic stimulation for unilateral spatial neglect in stroke patients. J Neurol Sci 359:219–225
Cha HG, Kim MK (2015) The effects of repetitive transcranial magnetic stimulation on unilateral neglect of acute stroke patients: a randomised controlled trial. Hong Kong Physiother J 33:53–58
Kim BR et al (2013) Effect of high- and low-frequency repetitive transcranial magnetic stimulation on visuospatial neglect in patients with acute stroke: a double-blind, sham-controlled trial. Arch Phys Med Rehabil 94:803–807
Cazzoli D et al (2012) Theta burst stimulation reduces disability during the activities of daily living in spatial neglect. Brain 135:3426–3439
Koch G et al (2012) θ-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology 78:24–30
Boasquevisque DS et al (2021) Contralesional cathodal transcranial direct current stimulation does not enhance upper limb function in subacute stroke: a pilot randomized clinical trial. Neural Plast 2021:8858394
Shaker HA et al (2018) Effect of transcranial direct current stimulation on cognitive function in stroke patients. Egypt J Neurol Psychiatr Neurosurg 54:32
Khaksarian M et al (2018) Anodal transcranial direct current stimulation enhances positive changes in movement functions, visual attention and depression of patients with chronic ischemic stroke: a clinical trial. Biomed Res Therapy 5:2841–2849
Yi YG et al (2016) The effect of transcranial direct current stimulation on neglect syndrome in stroke patients. Ann Rehabil Med 40:223–229
Kim K-U, Kim S-H, An T-G (2016) Effect of transcranial direct current stimulation on visual perception function and performance capability of activities of daily living in stroke patients. J Phys Ther Sci 28:2572–2575
Yun GJ, Chun MH, Kim BR (2015) The effects of transcranial direct-current stimulation on cognition in stroke patients. J Stroke 17:354–358
Sunwoo H et al (2013) Effects of dual transcranial direct current stimulation on post-stroke unilateral visuospatial neglect. Neurosci Lett 554:94–98
Takeuchi N et al (2009) Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med 41:1049–1054
Elsner B et al (2017) Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. J Neuroeng Rehabil 14:95
Nejati V, Salehinejad MA, Nitsche MA (2018) Interaction of the left dorsolateral prefrontal cortex (l-DLPFC) and right orbitofrontal cortex (OFC) in hot and cold executive functions: evidence from transcranial direct current stimulation (tDCS). Neuroscience 369:109–123
Webler RD et al (2022) DLPFC stimulation alters working memory related activations and performance: an interleaved TMS-fMRI study. Brain Stimul 15:823–832
Tomeh A, Yusof Khan AHK, Wan Sulaiman WA (2022) Repetitive transcranial magnetic stimulation of the primary motor cortex in stroke survivors-more than motor rehabilitation: a mini-review. Front Aging Neurosci 14:897837
Vigneau M et al (2006) Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30:1414–1432
Leff AP et al (2009) The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain J Neurol 132:3401–3410
Boukrina O et al (2022) Brain network dysfunction in poststroke delirium and spatial neglect: an fMRI study. Stroke 53:930–938
Corbetta M, Shulman GL (2011) Spatial neglect and attention networks. Annu Rev Neurosci 34:569–599
Funding
This study was supported by grants from the National Key R&D Program of China (2020YFC2004200) and Chongqing Talent Plan (cstc2022ycjh-bgzxm0184).
Author information
Authors and Affiliations
Contributions
Concept formation and study design were contributed by MY and JL. The screening of studies was independently conducted by JL and MY. Data extraction was performed by JL and MY. Examination and statistical analysis were contributed by JL, YG and MY. The methodology of the interventions and data interpretation were overseen by QH and JL. Risk of bias assessment and manuscript drafting were carried out by JS, XW, JL and MY. The entire study was supervised by YL and WY.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no potential competing interests.
Ethical approval
Our study did not require ethical board approval because our results came from randomized controlled trials, based on publicly available information without reference to the patient's private information.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, M., Liu, J., Guo, Y. et al. Comparative efficacy of non-invasive brain stimulation for post-stroke cognitive impairment: a network meta-analysis. Aging Clin Exp Res 36, 37 (2024). https://doi.org/10.1007/s40520-023-02662-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-023-02662-x