Abstract
Background
The risk of subsequent vertebral fractures (SVF) after the primary vertebral fracture cannot be explained by lower bone mineral density (BMD) alone. Computed tomography (CT) measurements of paravertebral muscle density (PMD) are recognized radiographic markers used to predict physical function, fragile fractures.
Aims
This study aims to investigate the relationship between PMD and the risk of SVF in cohorts of postmenopausal women, and to determine if combining both PMD and BMD measures derived from CT can improve the accuracy of predicting SVF.
Methods
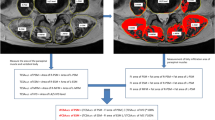
This study enrolled 305 postmenopausal women between the ages of 50 and 88 for 3 years of follow-up studies. Trabecular attenuation (Hounsfield units, HU) was measured at L1 level and muscle attenuation of paravertebral muscle at L3 level on preoperative lumbar CT scans to determine the L1 BMD and L3 PMD. Kaplan–Meier analysis was applied to evaluate SVF-free survival. The hazard ratios (HRs) of PMD for SVF events were estimated with the Cox proportional hazards model. The predictive values of L1 BMD and L3 PMD for SVF were quantified using the Receiver-Operating Characteristic (ROC) curve.
Result
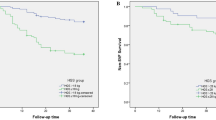
During the 3 years of follow-up studies, 88 patients (28.9%) suffered an SVF. ROC curve analysis demonstrated that an L3 PMD threshold of 32 HU had a sensitivity of 89.8% and a specificity of 62% for the prediction of SVF. Kaplan–Meier analysis showed that L3 PMD ≤ 32 HU was significantly associated with lower SVF-free survival (p < 0.001; log-rank test). After adjusting for age, BMI, diabetes, postoperative osteoporosis treatment, handgrip strength, L1 BMD, multivariate analyses also indicated a persistent modest effect of L3 PMD on SVF-free survival. The area under the ROC curve of L3 PMD and L1 BMD, combined to predict the risk of SVF, was 0.790, which was significantly higher than the value for L1 BMD alone (0.735). L3 PMD and L1 BMD significantly improved the accuracy of SVF risk prediction compared with L1 BMD alone, which was confirmed by reclassification improvement measures. The inclusion of handgrip strength and postoperative osteoporosis treatment in the model further improved SVF prediction accuracy, and PMD remained significant in the model.
Conclusion
Decreased L3 PMD is an independent risk predictor of SVF. Combined CT-based L1 BMD and L3 PMD can significantly improve the accuracy of predicting the risk of SVF in postmenopausal women who have suffered prior osteoporotic vertebral fractures.




Similar content being viewed by others
References
Zhu RS, Kan SL, Ning GZ et al (2019) Which is the best treatment of osteoporotic vertebral compression fractures: balloon kyphoplasty, percutaneous vertebroplasty, or non-surgical treatment? A Bayesian network meta-analysis. Osteoporos Int 30:287–298. https://doi.org/10.1007/s00198-018-4804-2
Lee HJ, Park J, Lee IW et al (2019) Clinical, Radiographic, and morphometric risk factors for adjacent and remote vertebral compression fractures over a minimum follow-up of 4 years after percutaneous vertebroplasty for osteoporotic vertebral compression fractures: novel three-dimensional voxel-based morphometric analysis. World neurosurgery 125:e146–e157. https://doi.org/10.1016/j.wneu.2019.01.020
Camacho PM, Petak SM, Binkley N et al (2020) American association of clinical endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract 26:1–46. https://doi.org/10.4158/GL-2020-0524SUPPL
Malik AT, Retchin S, Phillips FM et al (2020) Declining trend in osteoporosis management and screening following vertebral compression fractures—a national analysis of commercial insurance and medicare advantage beneficiaries. Spine J 20:538–546. https://doi.org/10.1016/j.spinee.2019.10.020
Hinde K, Maingard J, Hirsch JA et al (2020) Mortality outcomes of vertebral augmentation (vertebroplasty and/or balloon kyphoplasty) for osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Radiology 295:96–103. https://doi.org/10.1148/radiol.2020191294
Jang S, Graffy PM, Ziemlewicz TJ et al (2019) Opportunistic osteoporosis screening at routine abdominal and thoracic ct: normative L1 trabecular attenuation values in more than 20000 adults. Radiology 291:360–367. https://doi.org/10.1148/radiol.2019181648
Cheng X, Zhao K, Zha X et al (2021) Opportunistic screening using low-dose CT and the prevalence of osteoporosis in china: a nationwide. Multicenter Study J Bone Miner Res 36:427–435. https://doi.org/10.1002/jbmr.4187
Wang H, Zou D, Sun Z et al (2020) Hounsfield unit for assessing vertebral bone quality and asymmetrical vertebral degeneration in degenerative lumbar scoliosis. Spine (Phila Pa 1976) 45:1559–1566
Graffy PM, Lee SJ, Ziemlewicz TJ et al (2017) Prevalence of vertebral compression fractures on routine ct scans according to l1 trabecular attenuation: determining relevant thresholds for opportunistic osteoporosis screening. AJR Am J Roentgenol 209:491–496. https://doi.org/10.2214/AJR.17.17853
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31:1539–1547. https://doi.org/10.1200/JCO.2012.45.2722
Aubrey J, Esfandiari N, Baracos VE et al (2014) Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 210:489–497. https://doi.org/10.1111/apha.12224
Anderson DE, Quinn E, Parker E et al (2016) Associations of computed tomography-based trunk muscle size and density with balance and falls in older adults. J Gerontol A Biol Sci Med Sci 71:811–816. https://doi.org/10.1093/gerona/glv185
Wang L, Yin L, Zhao Y et al (2020) Muscle density, but not size, correlates well with muscle strength and physical performance. J Am Med Dir Assoc 22:751-759.e2. https://doi.org/10.1016/j.jamda.2020.06.052
Hirschfeld HP, Kinsella R, Duque G (2017) Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 28:2781–2790. https://doi.org/10.1007/s00198-017-4151-8
Scott D, Seibel M, Cumming R et al (2019) Does combined osteopenia/osteoporosis and sarcopenia confer greater risk of falls and fracture than either condition alone in older men? The concord health and ageing in men project. J Gerontol A Biol Sci Med Sci 74:827–834. https://doi.org/10.1093/gerona/gly162
Yin L, Xu Z, Wang L et al (2020) Associations of muscle size and density with proximal femur bone in a community dwelling older population. Front Endocrinol 11:503. https://doi.org/10.3389/fendo.2020.00503
Lang T, Cauley JA, Tylavsky F et al (2010) Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 25:513–519. https://doi.org/10.1359/jbmr.090807
Newman AB, Kupelian V, Visser M et al (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci 61:72–77. https://doi.org/10.1093/gerona/61.1.72
Wang L, Yin L, Zhao Y et al (2020) Muscle density discriminates hip fracture better than CTXA hip areal bone mineral density. J Cachexia Sarcopenia Muscle 11:1799–1812. https://doi.org/10.1002/jcsm.12616
Mourtzakis M, Prado CM, Lieffers JR et al (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33:997–1006. https://doi.org/10.1139/H08-075
Di Monaco M, Castiglioni C, De Toma E et al (2014) Handgrip strength but not appendicular lean mass is an independent predictor of functional outcome in hip—fracture women: a short-term prospective study. Arch Phys Med Rehabil 95:1719–1724. https://doi.org/10.1016/j.apmr.2014.04.003
Genant HK, Wu CY, van Kuijk C et al (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
Bozdogan H (2000) Akaike’s information criterion and recent developments in information complexity. J Math Psychol 44:62–91. https://doi.org/10.1006/jmps.1999.1277
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Kennedy KF, Pencina MJ (2010) A sas macro to compute added predictive ability of new markers predicting a dichotomous outcome. In: SouthEeast SAS users group annual meeting proceedings 2010. http://analytics.ncsu.edu/sesug/2010/SDA07.Kennedy.pdf.
Pencina MJ, D’Agostino RB, Pencina KM et al (2012) Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol 176:473–481. https://doi.org/10.1093/aje/kws207
Che H, Breuil V, Cortet B et al (2019) Vertebral fractures cascade: potential causes and risk factors. Osteoporos Int 30:555–563. https://doi.org/10.1007/s00198-018-4793-1
Kobayashi T, Kaneko M, Narukawa M (2020) Influence of prevalent vertebral fracture on the correlation between change in lumbar spine bone mineral density and risk of new vertebral fracture: a meta-analysis of randomized clinical trials. Clin Drug Investig 40:15–23. https://doi.org/10.1007/s40261-019-00868-4
Kaufman JM, Palacios S, Silverman S et al (2013) An evaluation of the fracture risk assessment tool (FRAX®) as an indicator of treatment efficacy: the effects of bazedoxifene and raloxifene on vertebral, nonvertebral, and all clinical fractures as a function of baseline fracture risk assessed by FRAX®. Osteoporos Int 24:2561–2569. https://doi.org/10.1007/s00198-013-2341-6
Pickhardt PJ, Pooler BD, Lauder T et al (2013) Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 158:588–595. https://doi.org/10.7326/0003-4819-158-8-201304160-00003
Lee SJ, Graffy PM, Zea RD et al (2018) Future osteoporotic fracture risk related to lumbar vertebral trabecular attenuation measured at routine body CT. J Bone Miner Res 33:860–867. https://doi.org/10.1002/jbmr.3383
Zhang SB, Xu HW, Yi YY et al (2021) Evaluation of the use of CT attenuation for the prediction of subsequent vertebral fracture in patients with osteoporosis. Pain Physician 24:E493-e500
Guizelini PC, de Aguiar RA, Denadai BS et al (2018) Effect of resistance training on muscle strength and rate of force development in healthy older adults: a systematic review and meta-analysis. Exp Gerontol 102:51–58. https://doi.org/10.1016/j.exger.2017.11.020
Peng X, Li X, Xu Z et al (2020) Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 Chinese females. Quant Imaging Med Surg 10:1590–1601. https://doi.org/10.21037/qims-19-835
Lorbergs AL, Allaire BT, Yang L et al (2019) A Longitudinal study of trunk muscle properties and severity of thoracic kyphosis in women and men: the Framingham study. J Gerontol A Biol Sci Med Sci 74:420–427. https://doi.org/10.1093/gerona/gly056
Huang CWC, Tseng IJ, Yang SW et al (2019) Lumbar muscle volume in postmenopausal women with osteoporotic compression fractures: quantitative measurement using MRI. Eur Radiol 29:4999–5006. https://doi.org/10.1007/s00330-019-06034-w
Wang WF, Lin CW, Xie CN et al (2019) The association between sarcopenia and osteoporotic vertebral compression refractures. Osteoporos Int 30:2459–2467. https://doi.org/10.1007/s00198-019-05144-x
Scott D, Chandrasekara SD, Laslett LL et al (2016) Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5–10 years in community-dwelling older adults. Calcif Tissue Int 99:30–42. https://doi.org/10.1007/s00223-016-0123-9
Zhang SB, Chen H, Xu HW et al (2020) Association between handgrip strength and subsequent vertebral-fracture risk following percutaneous vertebral augmentation. J Bone Miner Metab 39:186–192. https://doi.org/10.1007/s00774-020-01131-z
Kirk B, Feehan J, Lombardi G et al (2020) Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep 18:388–400. https://doi.org/10.1007/s11914-020-00599-y
Michaud M, Balardy L, Moulis G et al (2013) Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc 14:877–882. https://doi.org/10.1016/j.jamda.2013.05.009
Frank-Wilson AW, Farthing JP, Chilibeck PD et al (2016) Lower leg muscle density is independently associated with fall status in community-dwelling older adults. Osteoporos Int 27:2231–2240. https://doi.org/10.1007/s00198-016-3514-x
Bassani T, Casaroli G, Galbusera F (2019) Dependence of lumbar loads on spinopelvic sagittal alignment: An evaluation based on musculoskeletal modeling. PLoS ONE 14:e0207997. https://doi.org/10.1371/journal.pone.0207997
Kanis JA, Harvey NC, Cooper C et al (2016) A systematic review of intervention thresholds based on FRAX: a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos 11:25. https://doi.org/10.1007/s11657-016-0278-z
Harvey NC, Odén A, Orwoll E et al (2018) Measures of physical performance and muscle strength as predictors of fracture risk independent of FRAX, falls, and aBMD: a meta-analysis of the osteoporotic fractures in men (MrOS) study. J Bone Miner Res 33:2150–2157. https://doi.org/10.1002/jbmr.3556
Acknowledgements
Primary Funding Source: This work was funded by the Special Program for Research on Healthy Aging of Shanghai Municipal Health Commission (2020YJZX0116), and Science and Technology project of Jiangxi Provincial Health Commission (202140997).
Funding
The work of Shan-Jin Wang was supported by The Special Program for Research on Healthy Aging of Shanghai Municipal Health Commission under Grant 2020YJZX0116, and Science and Technology Project of Jiangxi Provincial Health Commission under Grant 202140997.
Author information
Authors and Affiliations
Contributions
SJW initiated the idea and did the data analysis. SBZ wrote the assay. YYY and HWX supervised and reviewed the manuscript. HC and XYF gathered the data and helped with the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The study does not present any potential conficts.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards declaration and its later amendments or comparable ethical standards.
Consent to participate
All patients agreed to participate in the study and signed an informed consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, SB., Chen, H., Xu, HW. et al. Computed tomography-based paravertebral muscle density predicts subsequent vertebral fracture risks independently of bone mineral density in postmenopausal women following percutaneous vertebral augmentation. Aging Clin Exp Res 34, 2797–2805 (2022). https://doi.org/10.1007/s40520-022-02218-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02218-5