Abstract
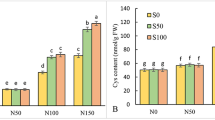
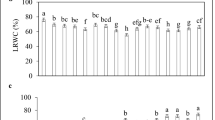
Elevated concentrations of salt induce ionic and osmotic stress, hindering plant development and growth through impairing various crucial metabolic functions. The current study was conducted to fathom how nitric oxide (NO) induces salinity stress tolerance in Lupinus termis L. plants. Varying concentrations of sodium chloride (0.0, 75 and 150 mM) were introduced to plants with/without supplementation of NO (in the form of sodium nitroprusside, 0.4 and 0.6 mM) to detect the associated metabolomic changes. Growth parameters, oxidative markers (lipid peroxidation, H2O2, and electrolyte leakage), photosynthetic pigments, phytohormonal content, certain osmoprotectants (organic acids contents, proline, soluble sugars, and polysaccharides), and mineral ions (Na+, Ca2+, K+, Mg2+) were determined in both shoots and roots. NO exogenous application significantly diminished the salinity-induced oxidative stress and alleviated salt’s inhibitory effect on lupine growth and photosynthetic pigment content. The most effective SNP (sodium nitroprusside) concentration in boosting salt stress tolerance in lupine was 0.4 mM. This could be correlated to the found upregulation of osmoprotectant molecules including soluble sugars, polysaccharides, and organic acids, namely malic, succinic, formic, acetic, and butyric acids, besides increased endogenous hormones level. In addition to the increased divalent cations (Ca2+ and Mg2+) content, reduced Na+ intake, and increased K+ influx in lupine plants supplemented with 0.4 mM SNP was evident.

Similar content being viewed by others
References
Ahmad, P., Abdel Latef, A. A., Hashem, A., Abd-Allah, E. F., Gucel, S., & Tran, L. P. (2016). Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2016.00347
Alexieva, V., Sergiev, I., Mapelli, S., & Karanov, E. (2001). The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell and Environment, 24, 1337–1344.
Amiri, J., & Eshghi, S. (2015). Ion and mineral concentrations in roots and leaves of two grapevine cultivars as affected by nitric oxide foliar application under NaCl stress. Journal International Des Sciences De La Vigne, 49, 155–164.
Ashraf, M., & Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, 59, 206–216.
Ashraf, M., & Waheed, A. (1990). Screening of local exotic accensions of lentil (Lens culinaris) for salt tolerance at two growth stages. Plant and Soil, 128, 167–176.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 39, 205–207.
Bonab, R. B., Saadatmand, S., Nazemiyeh, H., & Bakhsh, A. R. I. (2015). Alleviation effects of nitric oxide on the growth rate and photosynthetic pigments and reducing sugar content in NaCl-stressed coriander (Coriandrumsativum L.). Journal of Applied Environmental and Biological Sciences, 5, 577–585.
Chen, F., Wang, F., Sun, H. Y., Cai, Y., Mao, W. H., Zhang, G. P., Vincze, E., & Wu, F. B. (2010). Genotype-dependent effect of exogenous nitric oxide on Cd-induced changes in antioxidative metabolism, ultrastructure, and photosynthetic performance in barley seedlings (Hordeum vulgare). Journal of Plant Growth Regulation, 29, 394–408.
Chen, J., Xiong, D. Y., Wang, W. H., Hu, W. J., Simon, M., Xiao, Q., et al. (2013). Nitric oxide mediates root K+/Na+ balance in a mangrove plant, Kandelia obovata, by enhancing the expression of AKT1-type K+ channel and Na+/H+ antiporter under high salinity. PLoS ONE, 8, e71543.
Crawford, N. M., & Guo, F. Q. (2005). New insights into nitric oxide metabolism and regulatory functions. Trends in Plant Science, 10, 195–200.
Debez, A., Chaibi, W., & Bouzid, S. (2001). Effect du NaCl et de regulatoeurs de croissance sur la germination d’ Atriplex halimus L. Cahiers Agricultures, 10, 135–138.
Dionisio-Sese, M. L., & Tobita, S. (1998). Antioxidant responses of rice seedlings to salinity stress. Plant Science, 135, 1–9.
Esmail, N. Y., Hashem, H. A., & Hassanein, A. A. (2018). Effect of treatment with different concentrations of sodium nitroprusside on survival, germination, growth, photosynthetic pigments and endogenous nitric oxide content of Lupines termis L. Plants. Acta Scientific Agriculture, 25, 48–52.
Fan, H., Guo, S., Jiao, Y., Zhang, R., & Li, J. (2007). Effects of exogenous nitric oxide on growth, active oxygen species metabolism, and photosynthetic characteristics in cucumber seedlings under NaCl stress. Frontiers of Agriculture in China, 1, 308–314.
Farooq, M., Basra, S. M. A., Wahid, A., & Rehman, H. (2009). Exogenously applied nitric oxide enhances the drought tolerance in fine grain aromatic rice (Oryza sativa L.). Journal of Agronomy and Crop Science, 195, 254–261. https://doi.org/10.1111/j.1439-037X.2009.00367.x
Fatma, M., Masood, A., Per, T. S., & Khan, N. A. (2016). Nitric oxide alleviates salt stress inhibited photosynthetic response by interacting with sulfur assimilation in mustard. Frontiers in Plant Science, 7, 521. https://doi.org/10.3389/fpls.2016.00521
Feng, J., Wang, C., Chen, Q., Chen, H., Ren, B., Li, X., & Zuo, J. (2013). S-nitrosylation of phosphotransferase proteins represses cytokinin signaling. Nature Communications, 4, 1529. https://doi.org/10.1038/ncomms2541
Floryszak-Wieczorek, J., Arasimowicz, M., Milczarek, G., Jelen, H., & Jackowiak, H. (2007). Only an early nitric oxide burst, and the following wave of secondary nitric oxide generation enhanced effective defence responses of pelargonium to a necrotrophic pathogen. New Phytologist, 175, 718–730.
Ford, C. W., & Wilson, J. R. (1981). Changes in levels of solutes during osmotic adjustment to water stress in leaves of four tropical pasture species. Australian Journal of Plant Physiology, 8(1), 77–91.
Freschi, L. (2013). Nitric oxide and phytohormone interactions: Current status and perspectives. Frontiers in Plant Science, 4, 398. https://doi.org/10.3389/fpls.2013.00398
García-Mata, C., & Lamattina, L. (2002). Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiology, 128, 790–792.
Hasanuzzaman, M., Nahar, K., Alam, M. M., Roychowdhury, R., & Fujita, M. (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. International Journal of Molecular Sciences, 14, 9643–9684.
Hasegawa, P., Bressan, R. A., Zhu, J. K., & Bohnert, H. J. (2000). Plant cellular and molecular responses to high salinity. Annual Reviews of Plant Physiology and Plant Molecular Biology, 51, 463–499.
Hashem, H. A. (2006) Physiological and molecular actions of jasmonic acid on soybean plant. Ph.D. Thesis, Faculty of Science, Ain Shams University, Cairo, Egypt.
Hashem, H. A., Hassanein, A. A., & Esmail, N. Y. (2018). Nitric oxide enhances the adaptive responses of lupine plants against heavy-metal stress. Australian Journal of Crop Science, 12(12), 1962–1974.
Hayat, S., Mori, M., Pichtel, J., & Ahmad, A. (2010). Nitric oxide in plant physiology. Wiley Blackwell.
Hodges, D. M., DeLong, J. M., Forney, C. F., & Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604–611.
Homme, P. M., Gonalez, B., & Billard, J. (1992). Carbohydrate content, frutane and sucrose enzyme activities in roots, stubble and leaves of ryegrass (Lolium perenne L.) as affected by sources/link modification after cutting. Journal of Plant Physiology, 140, 282–291.
Hyang, L. E., Dae, K. H., & Seung, K. L. (2009). Nitric oxide reduced chlorophyll degradation in broccoli (Brassica oleracea L. var. italica) florets during senescence. Food Science and Technology International, 15, 223–228.
Javid, M. G., Sorooshzadeh, A., Moradi, F., Sanavy, S. A., & Allahdadi, I. (2011). The role of phytohormones in alleviating salt stress in crop plants. Australian Journal of Crop Science, 5, 726–734.
Keyster, M., Klein, A., & Ludidi, N. (2012). Caspase-like enzymatic activity and the ascorbate-glutathione cycle participate in salt stress tolerance of maize conferred by exogenously applied nitric oxide. Plant Signaling & Behavior, 7, 349–360.
Kopyra, M., & Gwozdz, E. A. (2004). The role of nitric oxide in plant growth regulation and responses to abiotic stresses. Acta Physiologia Plantarum, 26, 459–472.
Kramell, R.(1996). HPLC separation of jasmonic acid methyl ester enantiomers. Phytochemical analysis, 7, 738–743.
Leon, J., & Lozano-Juste, J. (2011). Nitric oxide regulates DELLA content and PIF expression to promote photomorphogenesis in Arabidopsis. Plant Physiology, 156, 1410–1423.
Li, Q. Y., Niu, H. B., Yin, J., Wang, M. B., Shao, H. B., Deng, D. Z., Chen, X. X., Ren, J. P., & Li, Y. C. (2008). Protective role of exogenous nitric oxide against oxidative stress induced by salt stress in barley (Hordeum vulgare). Colloids and Surface B., 65, 220–225.
Liang, Y. C., Chen, Q., Liu, Q., Zhang, W. H., & Ding, R. X. (2003). Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). Journal of Plant Physiology, 160, 1157–1164.
Lucas, M. M., Stoddard, F. L., Annicchiarico, P., Frias, J., Martinez-Villaluenga, C., & Sussmann, D. (2015). The future of lupin as a protein crop in Europe. Frontiers in Plant Science, 6, 705.
Mahajan, S., & Tuteja, N. (2005). Cold, salinity and drought stresses: An overview. Archives of Biochemistry and Biophysics, 444, 139–158.
Manai, J., Zaghdoud, C., Debouba, M., Donia, H. G. & Bouthour (2012). Role of nitric oxide in saline stress: Implications on nitrogen metabolism. African Journal of Plant Science, 6, 376–382.
Mangano, S., Silberstein, S., & Santa-María, G. E. (2008). Point mutations in the barley HvHAK1 potassium transporter lead to improved K+-nutrition and enhanced resistance to salt stress. FEBS Letters, 582, 3922–3928.
Marounek, M., Skřivanova, E., & Skřivanova, V. (2006). Selenium content and antioxidant status in tissues of veal calves fed a diet supplemented with selenium yeast. Slovak Journal of Animal Science, 39, 51–54.
Metzner, H., Rau, H., & Senger, H. (1965). Studies on the synchronisability of individual pigment deficient mutants of Chlorella. Planta, 65, 186–194.
Munns, R. (2002). Comparative physiology of salt and water stress. Plant, Cell and Environment, 25, 239–250.
Pantin, F., Renaud, J., Barbier, F., Vavasseur, A., Le Thiec, D., Rose, C., Bariac, T., Casson, S., McLachlan, D.H., Hetherington, A.M., Muller, B., & Simonneau, T. (2013). Developmental priming of stomatal sensitivity to abscisic acid by leaf microclimate. Current Biology, 23(18), 1805–11. https://doi.org/10.1016/j.cub.2013.07.050.
Plustea, L., Negrea, M., Cocan, I., Radulov, I., Tulcan, C., Berbecea, A., Popescu, I., Obistioiu, D., Hotea, I., Suster, G., Boeriu, A. E., & Alexa, E. (2022). Lupin (Lupinus spp.)-fortified bread: A sustainable, nutritionally, functionally, and technologically valuable solution for bakery. Foods., 11(14), 2067. https://doi.org/10.3390/foods11142067. PMID: 35885310; PMCID: PMC9316204.
Popova, L., & Tuan, T. (2010). Nitric oxide in plants: Properties, biosynthesis and physiological functions. Iranian Journal of Science and Technology Transactions A, 34, 173–183.
Rains, D. W., Croughan, T. P., & Stavarek, S. J. (1980). Selection of salt-tolerant plants using tissue culture. In D. W. Rains, R. C. Valentine, & A. Hollaender (Eds.), Genetic engineering of osmoregulation. Basic life sciences. (Vol. 14). Boston, MA: Springer. https://doi.org/10.1007/978-1-4684-3725-6_19
Sakhabutdinova, A. R., Fatkhutdinova, D. R., Bezrukova, M. V., & Shakirova, F. M. (2003). Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulgarian Journal of Plant PhysioloGy, 29, 314–319.
Sassi, S., Aydi, S., Gonzalez, E. M., Arrese-Igor, C., & Abdelly, C. (2010). Understanding osmotic stress tolerance in leaves and nodules of two Phaseolus vulgaris cultivars with contrasting drought tolerance. Symbiosis, 52(1), 1.
Shabala, S., & Pottosin, I. (2014). Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiologia Plantarum, 151, 257–279.
Sharma, A., Kapoor, D., Wnag, J., Landi, M., Zheng, D., Yan, D., & Yuan, H. (2020). Nitric oxide mechanisms adopted by plants to cope with salinity. Biologia Plantarum, 64, 512–518.
Sheokand, S., Bhankar, V., & Sawhney, V. (2010). Ameliorative effect of exogenous nitric oxide on oxidative metabolism in NaCl treated chickpea plants. Brazilian Journal of Plant Physiology, 22, 81–90.
Shi, Q., Ding, F., Wang, X., & Wei, M. (2007). Exogenous nitric oxide protects cucumber roots against oxidative stress induced by salt stress. Plant Physiology and Biochemistry, 45, 542–550.
Shi, Q., & Zhu, Z. (2008). Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. Environmental and Experimental Botany, 63, 317–326.
Shindy, W. W., & Smith, O. (1975). Identification of plant hormones from cotton ovules. Plant Physiology, 55, 550–554.
Sivritepe, N., Sivritepe, H. O., Celik, H., & Katkat, A. V. (2010). Salinity responses of grafted grapevines: Effects of scion and rootstock genotypes. Notulae Botanicae Horti Agrobotanici Cluj, 38, 193–201.
Snedecor, G. W., & Cochran, W. G. (1980). Statistical Methods (7th ed.). Iowa State University Press.
Steuer, R., Nesi, A. N., Fernie, A. R., Gross, T., Blasius, B., & Selbig, J. (2007). From structure to dynamics of metabolic pathways: Application to the plant mitochondrial TCA cycle. Bioinformatics, 23(11), 1378–1385.
Tan, J., Zhao, H., Hong, J., Han, Y., Li, H., & Zhao, W. (2008). Effects of exogenous nitric oxide on photosynthesis, antioxidant capacity and proline accumulation in wheat seedlings subjected to osmotic stress. World Journal of Agriculture Science, 4, 307–313.
Terrile, M. C., Parıś, R., Calderón-Villalobos, L. I. A., Iglesias, M. J., Lamattina, L., Estelle, M., & Casalonque, C. A. (2012). Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis Transport inhibitor response 1 auxin receptor. Plant Journal, 70, 492–500.
Tester, M., & Davenport, M. (2003). Na+ tolerance and Na+ transport in higher plants. Annals of Botany, 91(5), 503–527. https://doi.org/10.1093/aob/mcg058
Uchida, A., Jagendorf, A. T., Hibino, T., & Takabe, T. (2002). Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Science, 163, 515–523.
Umezawa, T., Fujita, M., Fujita, Y., Yamaguchi-Shinozaki, K., & Shinozaki, K. (2006). Engineering drought tolerance in plants: Discovering and tailoring genes to unlock the future. Current Opinion in Biotechnology, 17(2), 113–122.
Vogel, A. J. (1975). A textbook of practical organic chemistry (3rd ed., pp. 483–485). English Language Book Society and Longman Group Ltd.
Wang, Y., Mopper, S., & Hasentein, K. H. (2001). Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. Journal of Chemical Ecology, 27, 327–342.
Wendehenne, D., Pugin, A., Klessing, D. F., & Durner, J. (2001). Nitric oxide: Comparative synthesis and signaling in animal and plant cells. Trends in Plant Science, 6, 177–183.
Whistler, R. L., Wolform, M. L., BeMiller, J. N. & Shafizadeh, F. (1962): Anthrone colorimetric method. In: Methods in Carbohydrate Chemistry (vol. 1, pp. 384). New York, London: Academic Press.
Wodecki, Z., Torlop, B., & Slebioda, M. (1991). Chromatographic determination of citric acid for monitoring the mold process. Journal of Chromatography, 558, 302–305.
Wu, X., Zhu, W., Zhang, H., Ding, H., & Zhang, H. J. (2011). Exogenous nitric oxide protects against salt induced oxidative stress in the leaves from two genotypes of tomato (Lycopersicom esculentum Mill.). Acta Physiologia Plantarum, 33, 1199–1209.
Xu, J., Wang, W., Yin, H., Liu, X., Sun, H., & Mi, Q. (2010). Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant and Soil, 326, 321–330.
Yasar, F., Kusvuran, S., & Ellialtıolu, S. (2006). Determination of antioxidant activities in some melon (Cucumis melo L.) varieties and cultivars under salt stress. Journal of Horticultural Science and Biotechnology, 81(4), 627–630.
Yin, Y., Li, S., Liao, W., Lu, Q., Wen, X., & Lu, C. (2010). Photosystem II photochemistry, photoinhibition, and the xanthophylls cycle in heat-stressed rice leaves. Journal of Plant Physiology, 167, 959–966.
Zhang, Y., Wang, L., Liu, Y., Zhang, Q., Wei, Q., & Zhang, W. (2006). Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta, 224, 545–555.
Zhao, L., Zhang, F., Guo, J., Yang, Y., Li, B., & Zhang, L. (2004). Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiology, 134, 849–857.
Zhao, M. G., Tian, Q. Y., & Zhang, W. H. (2007). Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiology, 144, 206–217.
Zheng, C., Jiang, D., Liu, F., Dai, T., Liu, W., Jing, Q., & Cao, W. (2009). Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environmental and Experimental Botany, 67, 222–227.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hashem, H.A., Esmail, N.Y. & Hassanein, A.A. Physiological changes in lupine plants in response to salt stress and nitric oxide signal. Plant Physiol. Rep. 28, 299–311 (2023). https://doi.org/10.1007/s40502-023-00720-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-023-00720-0