Abstract
Purpose of Review
Microfluidics devices have garnered significant attention in response to animal rights concerns and due to faithful replication of in vivo conditions. In the realm of materials characterization, these chips offer the means to assess cell cytotoxicity and biocompatibility, effectively emulating the cellular microenvironment. Notably, there was a substantial temporal gap, roughly 16 years, between the first organ-on-a-chip (OOC) from the technology in the late 1990s and the development of the first dental microfluidic chip. To provide clarity on their usage, we classify dental microfluidic chips into distinct generations. Additionally, we present an overview of recent dental materials tested on chips over the past 5 years to examine the advantages and disadvantages of disposable materials for chip production, and address the limitations and challenges facing the dental research community.
Recent Findings
Recent studies have explored the application of microfluidics in dental materials and in particular adhesives, including HEMA, acid phosphoric, Adper Scotch bond, SE BOND, silver diamine fluoride, silicate cement, EDTA-chitosan, and emerging regenerative drugs. PDMS and PMMA are the primary materials choice for chip manufacturing, with a bottom-up approach often selected for cell injection.
Summary
The use of microfluidics for dental research is categorized into generations, but a disparity exists between prototype standardization and result validation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Joseph X, Akhil V, Arathi A, Mohanan PV. Comprehensive development in organ-on-a-chip technology. J Pharm Sci. 2022;111:18–31. https://doi.org/10.1016/j.xphs.2021.07.014.
Koyilot MC, Natarajan P, Hunt CR, Sivarajkumar S, Roy R, Joglekar S, Pandita S, Tong CW, Marakkar S, Subramanian L, Yadav SS, Cherian AV, Pandita TK, Shameer K, Yadav KK. Breakthroughs and applications of organ-on-a-chip technology. Cells. 2022;11:1828. https://doi.org/10.3390/cells11111828.
Akhtar A. The flaws and human harms of animal experimentation. Camb Q Healthc Ethics. 2015;24:407–19. https://doi.org/10.1017/S0963180115000079.
Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, Chen Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda). 2017;32:266–77. https://doi.org/10.1152/physiol.00036.2016.
Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–72. https://doi.org/10.1038/nbt.2989.
Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–60. https://doi.org/10.1038/nrd4539.
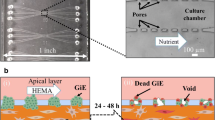
Ly KL, Rooholghodos SA, Rahimi C, Rahimi B, Bienek DR, Kaufman G, Raub CB, Luo X. An oral-mucosa-on-a-chip sensitively evaluates cell responses to dental monomers. Biomed Microdevices. 2021;23:7. https://doi.org/10.1007/s10544-021-00543-6.
Jodat YA, Kang MG, Kiaee K, Kim GJ, Martinez AFH, Rosenkranz A, Bae H, Shin SR. Human-derived organ-on-a-chip for personalized drug development. Curr Pharm Des. 2018;24:5471–86. https://doi.org/10.2174/1381612825666190308150055.
Bilitewski U, Genrich M, Kadow S, Mersal G. Biochemical analysis with microfluidic systems. Anal Bioanal Chem. 2003;377:556–69. https://doi.org/10.1007/s00216-003-2179-4.
Lignos I, Maceiczyk R, deMello AJ. Microfluidic technology: uncovering the mechanisms of nanocrystal nucleation and growth. Acc Chem Res. 2017;50:1248–57. https://doi.org/10.1021/acs.accounts.7b00088.
Wu J, He Z, Chen Q, Lin J-M. Biochemical analysis on microfluidic chips. TrAC Trends Anal Chem. 2016;80:213–31. https://doi.org/10.1016/j.trac.2016.03.013.
Orti V, Collart-Dutilleul PY, Piglionico S, Pall O, Cuisinier F, Panayotov I. Pulp regeneration concepts for nonvital teeth: from tissue engineering to clinical approaches. Tissue Eng Part B Rev. 2018;24:419–42. https://doi.org/10.1089/ten.TEB.2018.0073.
Lam RH, Cui X, Guo W, Thorsen T. High-throughput dental biofilm growth analysis for multiparametric microenvironmental biochemical conditions using microfluidics. Lab Chip. 2016;16:1652–62. https://doi.org/10.1039/c6lc00072j.
Kim J, Park HD, Chung S. Microfluidic approaches to bacterial biofilm formation. Molecules. 2012;17:9818–34. https://doi.org/10.3390/molecules17089818.
Song JL, Au KH, Huynh KT, Packman AI. Biofilm responses to smooth flow fields and chemical gradients in novel microfluidic flow cells. Biotechnol Bioeng. 2014;111:597–607. https://doi.org/10.1002/bit.2510.
Jeong HH, Jeong SG, Park A, Jang SC, Hong SG, Lee CS. Effect of temperature on biofilm formation by Antarctic marine bacteria in a microfluidic device. Anal Biochem. 2014;446:90–5. https://doi.org/10.1016/j.ab.2013.10.027.
Son M, Ahn SJ, Guo Q, Burne RA, Hagen SJ. Microfluidic study of competence regulation in Streptococcus mutans: environmental inputs modulate bimodal and unimodal expression of comX. Mol Microbiol. 2012;86:258–72. https://doi.org/10.1111/j.1365-2958.2012.08187.x.
Cui X, Yip HM, Zhu Q, Yang C, Lam RH. Microfluidic long-term differential oxygenation for bacterial growth characteristics analyses. RSC Adv. 2014;4:16662–21667. https://doi.org/10.1039/C4RA01577K.
Skolimowski M, Nielsen MW, Emnéus J, Molin S, Taboryski R, Sternberg C, Dufva M, Geschke O. Microfluidic dissolved oxygen gradient generator biochip as a useful tool in bacterial biofilm studies. Lab Chip. 2010;10:2162–9. https://doi.org/10.1039/c003558k.
Rahimi C, Rahimi B, Padova D, Rooholghodos SA, Bienek DR, Luo X, Kaufman G, Raub CB. Oral mucosa-on-a-chip to assess layer-specific responses to bacteria and dental materials. Biomicrofluidics. 2018;12:054106. https://doi.org/10.1063/1.5048938.
França CM, Tahayeri A, Rodrigues NS, Ferdosian S, Puppin Rontani RM, Sereda G, Ferracane JL, Bertassoni LE. The tooth on-a-chip: a microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip. 2020;20:405–13. https://doi.org/10.1039/c9lc00915a.
Vurat MT, Şeker Ş, Lalegül-Ülker Ö, Parmaksiz M, Elçin AE, Elçin YM. Development of a multicellular 3D-bioprinted microtissue model of human periodontal ligament-alveolar bone biointerface: towards a pre-clinical model of periodontal diseases and personalized periodontal tissue engineering. Genes Dis. 2022;9:1008–23. https://doi.org/10.1016/j.gendis.2020.11.011.
•• Rodrigues NS, França CM, Tahayeri A, Ren Z, Saboia VPA, Smith AJ, Ferracane JL, Koo H, Bertassoni LE. Biomaterial and biofilm interactions with the pulp-dentin complex-on-a-chip. J Dent Res. 2021;100:1136–43. https://doi.org/10.1177/0022034521101642.This paper brings the current dental materials used on clinical practice to in vitro testing on chip.
Hu S, Muniraj G, Mishra A, Hong K, Lum JL, Hong CHL, Rosa V, Sriram G. Characterization of silver diamine fluoride cytotoxicity using microfluidic tooth-on-a-chip and gingival equivalents. Dent Mater. 2022;38:1385–94. https://doi.org/10.1016/j.dental.2022.06.025.
•• Zhou Y, Zhao Y, Han J. EDTA-chitosan is a feasible conditioning agent for dentin bonding. Clin Oral Invest. 2022;26:3449–58. https://doi.org/10.1007/s00784-021-04270-3.This paper explores the tooth on chip tests correlated with mechanical tests to prove the efficiency of their new agent for dentin bonding.
•• Bordini EAF, Cassiano FB, Bronze-Uhle ES, Alamo L, Hebling J, de Souza Costa CA, Soares DG. Chitosan in association with osteogenic factors as a cell-homing platform for dentin regeneration: analysis in a pulp-in-a-chip model. Dent Mater. 2022;38:655–69. https://doi.org/10.1016/j.dental.2022.02.004.This paper is the first one to produce a scaffold for dentin regeneration and test inside a chip.
Liu X, Holzwarth JM, Ma PX. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci. 2012;12:911–9. https://doi.org/10.1002/mabi.201100466.
BaoLin G, Ma PX. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci China Chem. 2014;57:490–500. https://doi.org/10.1007/s11426-014-5086-y.
Pilipchuk SP, Monje A, Jiao Y, Hao J, Kruger L, Flanagan CL, Hollister SJ, Giannobile WV. Integration of 3D printed and micropatterned polycaprolactone scaffolds for guidance of oriented collagenous tissue formation in vivo. Adv Healthc Mater. 2016;5:676–87. https://doi.org/10.1002/adhm.201500758.
Lim SS, Chai CY, Loh HS. In vitro evaluation of osteoblast adhesion, proliferation and differentiation on chitosan-TiO2 nanotubes scaffolds with Ca2+ ions. Mater Sci Eng C Mater Biol Appl. 2017;76:144–52. https://doi.org/10.1016/j.msec.2017.03.075.
Tajeddin A, Mustafaoglu N. Design and fabrication of organ-on-chips: promises and challenges. Micromachines (Basel). 2021;12(12):1443. https://doi.org/10.3390/mi12121443.
Nielsen JB, Hanson RL, Almughamsi HM, Pang C, Fish TR, Woolley AT. Microfluidics: innovations in materials and their fabrication and functionalization. Anal Chem. 2020;92:150–68. https://doi.org/10.1021/acs.analchem.9b04986.
Campbell SB, Wu Q, Yazbeck J, Liu C, Okhovatian S, Radisic M. Beyond polydimethylsiloxane: alternative materials for fabrication of organ-on-a-chip devices and microphysiological systems. ACS Biomater Sci Eng. 2021;7:2880–99. https://doi.org/10.1021/acsbiomaterials.0c00640.
•• Nahak BK, Mishra A, Preetam S, Tiwari A. Advances in organ-on-a-chip materials and devices. ACS Appl Bio Mater. 2022;5:3576–607. https://doi.org/10.1021/acsabm.2c00041.This paper brilliantly describes the science of materials used as well as the main limitations of this technology.
Torino S, Corrado B, Iodice M, Coppola G. PDMS-based microfluidic devices for cell culture. Inventions. 2018;3:65. https://doi.org/10.3390/INVENTIONS3030065.
Hwang J, Cho YH, Park MS, et al. Microchannel fabrication on glass materials for microfluidic devices. Int J Precis Eng Manuf. 2019;20:479–95. https://doi.org/10.1007/s12541-019-00103-2.
Cong H, Zhang N. Perspectives in translating microfluidic devices from laboratory prototyping into scale-up production. Biomicrofluidics. 2022;16:021301. https://doi.org/10.1063/5.0079045.
Ren K, Zhou J, Wu H. Materials for microfluidic chip fabrication. Acc Chem Res. 2013;46:2396–406. https://doi.org/10.1021/ar300314s.
Wolf MP, Salieb-Beugelaar GB, Hunziker P. PDMS with designer functionalities—properties, modifications strategies, and applications. Prog Polym Sci. 2018;83:97–134. https://doi.org/10.1016/j.progpolymsci.2018.06.001.
McDonald JC, Whitesides GM. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc Chem Res. 2002;35:491–9. https://doi.org/10.1021/ar010110q.
McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJ, Whitesides GM. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis. 2000;21:27–40. https://doi.org/10.1002/(SICI)1522-2683(20000101)21:1%3c27::AID-ELPS27%3e3.0.CO;2-C.
Ren K, Chen Y, Wu H. New materials for microfluidics in biology. Curr Opin Biotechnol. 2014;25:78–85. https://doi.org/10.1016/j.copbio.2013.09.004.
Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–8. https://doi.org/10.1126/science.1188302.
Toepke MW, Beebe DJ. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip. 2006;6:1484–6. https://doi.org/10.1039/b612140c.
Winkler TE, Feil M, Stronkman EFGJ, Matthiesen I, Herland A. Low-cost microphysiological systems: feasibility study of a tape-based barrier-on-chip for small intestine modeling. Lab Chip. 2020;20:1212–26. https://doi.org/10.1039/d0lc00009d.
Lee JN, Park C, Whitesides GM. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem. 2003;75:6544–54. https://doi.org/10.1021/ac0346712.
Zhou J, Ellis AV, Voelcker NH. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis. 2010;31:2–16. https://doi.org/10.1002/elps.200900475.
Holczer E, Fürjes P. Effects of embedded surfactants on the surface properties of PDMS; applicability for autonomous microfluidic systems. Microfluid Nanofluid. 2017;21:81. https://doi.org/10.1007/s10404-017-1916-5.
Roman GT, Culbertson CT. Surface engineering of poly(dimethylsiloxane) microfluidic devices using transition metal sol-gel chemistry. Langmuir. 2006;22:4445–51. https://doi.org/10.1021/la053085w.
Slentz BE, Penner NA, Lugowska E, Regnier F. Nanoliter capillary electrochromatography columns based on collocated monolithic support structures molded in poly(dimethyl siloxane). Electrophoresis. 2001;22:3736–43. https://doi.org/10.1002/1522-2683(200109)22:17%3c3736::AID-ELPS3736%3e3.0.CO;2-Y.
Singh A, Malek CK, Kulkarni SK. Development in microreactor technology for nanoparticle synthesis. Int J Nanosci. 2010;9:93–112. https://doi.org/10.1142/S0219581X10006557.
Kotz F, Mader M, Dellen N, Risch P, Kick A, Helmer D, Rapp BE. Fused deposition modeling of microfluidic chips in polymethylmethacrylate. Micromachines (Basel). 2020;11:E873. https://doi.org/10.3390/mi11090873.
Nge PN, Rogers CI, Woolley AT. Advances in microfluidic materials, functions, integration, and applications. Chem Rev. 2013;113:2550–83. https://doi.org/10.1021/cr300337x.
•• Leung CM, de Haan P, Ronaldson-Bouchard K et al. A guide to the organ-on-a-chip.Nat Rev Methods Primers 2022;2:33. https://doi.org/10.1038/s43586-022-00118-6.This paper describes the main considerations of the organ on chip devices taking into account concepts of design fabrication, materials properties and the main applications of this dispositive.
• Leung CM, de Haan P, Ronaldson-Bouchard K et al. A guide to the organ-on-a-chip. Nat Rev Methods Primers 2022;2:33. https://doi.org/10.1038/s43586-022-00118-6.This paper describes the main considerations of the organ on chip devices taking into account concepts of design fabrication, materials properties and the main applications of this dispositive.
• Rogal J, Schlünder K, Loskill P. Developer’s guide to an organ-on-chip model. ACS Biomater Sci Eng. 2022;8(11):4643–7. https://doi.org/10.1021/acsbiomaterials.1c01536.This review has given an overview of the planning and development of organ on chip from a biological perspective.
Lee KS, Ram RJ. Plastic-PDMS bonding for high pressure hydrolytically stable active microfluidics. Lab Chip. 2009;9:1618–24. https://doi.org/10.1039/b820924c.
Koyilot MC, Natarajan P, Hunt CR, Sivarajkumar S, Roy R, Joglekar S, Pandita S, Tong CW, Marakkar S, Subramanian L, Yadav SS, Cherian AV, Pandita TK, Shameer K, Yadav KK. Breakthroughs and applications of organ-on-a-chip technology. Cells. 2022;11:1828. https://doi.org/10.3390/cells11111828.
Vunjak-Novakovic G, Ronaldson-Bouchard K, Radisic M. Organs-on-a-chip models for biological research. Cell. 2021;184:4597–611. https://doi.org/10.1016/j.cell.2021.08.005.
Herland A, Maoz BM, Das D, Somayaji MR, Prantil-Baun R, Novak R, Cronce M, Huffstater T, Jeanty SSF, Ingram M, Chalkiadaki A, Benson Chou D, Marquez S, Delahanty A, Jalili-Firoozinezhad S, Milton Y, Sontheimer-Phelps A, Swenor B, Levy O, Parker KK, Przekwas A, Ingber DE. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat Biomed Eng. 2020;4:421–36. https://doi.org/10.1038/s41551-019-0498-9.
Lee SW, Lee SS. Shrinkage ratio of PDMS and its alignment method for the wafer level process. Microsyst Technol. 2008;14:205–8. https://doi.org/10.1007/s00542-007-0417-y.
Funding
Authors appreciate the financial support offered by Coordination of Superior Level Staff Improvement (CAPES) grant no. AUX/CAPES/PROEX:88887.508828/2020–00.
Author information
Authors and Affiliations
Contributions
M.O., J.R.C. and R.M.P.R. conceived and planned the research. J.R.C. develop the research. J.R.C and R.M.P.R prepare the main manuscript text; J.R.C. prepared figure 1 and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camassari, J.R., Özcan, M. & Rontani, R.M.P. A Scoping Review on the Advent of Microfluidic Devices in Dentistry. Curr Oral Health Rep 11, 78–86 (2024). https://doi.org/10.1007/s40496-024-00365-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40496-024-00365-4