Abstract
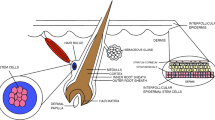
The skin is the largest organ of the body and performs various functions. Various stem cells are responsible for repairing damaged tissues and restoration of tissue proportion, which results in wound repair. If the wound healing process is not completed or impaired, it results in non-healing or delayed healing of wounds and known as chronic wounds. Sometimes, rupture of stem cells causes chronic wound by molecular and cellular actions in which the reason is not known. In the present review, we describe different types of stem cells for therapeutic application including adult mesenchymal stem cells, bone marrow stem cells, embryonic stem cells, umbilical cord stem cells, and induced pluripotent stem cells. Here, we enlighten on recent advances surrounding stem cell therapies for wound healing and their clinical acceptance criteria. We summarize an overview of the original function and therapeutic applications of the most promising stem cell populations for wound healing. Better therapeutic research on chronic wound healing is ongoing as current therapies have limited efficacy for this; adult stem cells can become good options for several skin pathologies. Keratinocytes are produced by epidermal stem cells. Due to the skin barrier properties of the keratinized layer through terminal differentiation caused by keratinocytes, it supports to the skin epidermis for regeneration. As stem cells can regenerate and distinguish the biomolecular location, it is gaining appreciation from three decades for the treatment of ulcers (venous) and diabetic chronic disease. This review also described various therapeutic approaches for the treatment of non-healing wounds and discussed about epidermal stem cell strategy, along with skin tissue engineering and follicle repair approach.



Similar content being viewed by others
References
Blitterswijk CA, Thomsen P. Tissue engineering. Amsterdam; Boston: Elsevier/Academic Press; 2008.
Harvey C. Wound healing. Orthop Nurs. 2005;24(2):143–57.
Atiyeh BS, Ioannovich J, Al-Amm CA, El-Musa KA. Management of acute and chronic open wounds: the importance of moist environment in optimal wound healing. Curr Pharm Biotechnol. 2002;3:179–95.
Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24.
Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35:171–80.
Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25:73–8.
Murphy PS, Evans GR. Advances in wound healing: a review of current wound healing products. Plast Surg Int. 2012;2012:190436.
Cronin H, Goldstein G. Biologic skin substitutes and their applications in dermatology. Dermatol Surg. 2013;39:30–4.
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62.
Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. Stem cell strategies in burns care. Burns. 2007;33:282–91.
Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–59.
Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res. 2009;67(3):253–71.
Cole-King A, Harding KG. Psychological factors and delayed healing in chronic wounds. Psychosom Med. 2001;63(2):216–20.
Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Investig Dermatol. 2007;127(3):514–25.
Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71.
Crovetti G, Martinelli G, Issi M, et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30(2):145–51.
Aarabi S, Longaker MT, Gurtner GC. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med. 2007;4(9):e234.
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46.
Heldin CH, Ostman A. Ligand-induced dimerization of growth factor receptors: variations on the theme. Cytokine Growth Factor Rev. 1996;7:3–10.
Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031–7.
Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006a;117:1e-S-32e-S.
Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006b;117:12S-34S.
Glat PM, Jelks GW, Jelks EB, et al. Evolution of the lateral canthoplasty: techniques and indications. Plast Reconstr Surg. 1997;100:1396–405.
Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9.
Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:72–140.
Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–42.
Werner S, Smola H, Liao X, et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994;266:819–22.
Niu J, Chang Z, Peng B, et al. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-kappaB transcription factors. J Biol Chem. 2007;282:6001–11.
Phillips SJ. Physiology of wound healing and surgical wound care. ASAIO J. 2000;46:S2–5.
Desmouliere A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995;19:471–6.
Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176:26S-38S.
Mignatti P, Rifkin DB. Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzyme Protein. 1996;49:117–37.
Jinnin M, Ihn H, Mimura Y, et al. Regulation of fibrogenic/fibrolytic genes by platelet-derived growth factor C, a novel growth factor, in human dermal fibroblasts. J Cell Physiol. 2005;202:510–7.
Clark RA. Biology of dermal wound repair. Dermatol Clin. 1993;11:647–66.
Cherubino M, Rubin JP, Miljkovic N, Kelmendi-Doko A, Marra KG. Adipose-derived stem cells for wound healing applications. Ann Plast Surg. 2011;66:210–5.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886.
Fernandez-Bances I, Perez-Basterrechea M, Perez-Lopez S, et al. Repair of long-bone pseudoarthrosis with autologous bone marrow mononuclear cells combined with allogenic bone graft. Cytotherapy. 2013;15:571–7.
Garcia-Gomez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10:1453–68.
Cihantimur B, Kahveci R, Ozcan M. Comparing Kaltostat with Jelonet in the treatment of split-thickness skin graft donor sites. Eur J Plastic Surg. 1997;20:260–3.
Davis JS. Story of plastic surgery. Ann Surg. 1941;113:651–6.
Akan M, Yildirim S, Misirlioglu A, Ulusoy G, Akoz T, Avci G. An alternative method to minimize pain in the split-thickness skin graft donor site. Plast Reconstr Surg. 2003;111(7):2243–9.
Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305–13.
Solovey P, Kyryk O, Barchuk V. Xenografts-liophilized pig skin as a burn wound cover. Burns. 2007;33:S85–6.
Robson MC, Barbul A. Guidelines for the best care of chronic wounds. Wound Repair Regen. 2006;14(6):647–8.
Gharaee-Kermani M, Phan SH. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des. 2001;7(11):1083–103.
Londahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic footulcers in patients with diabetes. Diabetes Care. 2010;33(5):998–1003.
Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22(5):569–78.
Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7(5):335–46.
Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, Carrillo-Poveda JM, Cuervo-Serrato B, Peláez-Gorrea P, Sopena-Juncosa JJ. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018;9(1):2–20.
Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24:16.
Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale JBiol Med. 2010;83:1–9.
Yung YL, Fu SC, Cheuk YC, Qin L, Ong MT, Chan KM, Yung PS. Optimisation of platelet concentrates therapy: composition, localisation, and duration of action. Asia Pac J SportsMed Arthrosc Rehabil Technol. 2017;7:27–36.
Babaei V, Afradi H, Gohardani HZ, Nasseri F, Azarafza M, Teimourian S. Management of chronic diabetic foot ulcers using platelet-rich plasma. J Wound Care. 2017;26:784–7.
Hersant B, SidAhmed-Mezi M, Bosc R, Meningaud JP. Autologous platelet-rich plasma/thrombin gel combined with split-thickness skin graft to manage postinfectious skin defects: a randomized controlled study. Adv Skin Wound Care. 2017;30:502–8.
Roubelakis MG, Trohatou O, Roubelakis A, Mili E, Kalaitzopoulos I, Papazoglou G, Pappa KI, Anagnou NP. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev. 2014;10:417–28.
Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, Lee Y. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23:424–31.
DeRossi R, Coelho AC, Mello GS, Frazilio FO, Leal CR, Facco GG, Brum KB. Effects of platelet-rich plasma gel on skin healing in surgical wound in horses. Acta Cir Bras. 2009;24:276–81.
Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–5.
Wu SC, Marston W, Armstrong DG. Wound care:the role of advanced wound-healing technologies. J Am Podiatr Med Assoc. 2010;100(5):385–94.
Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAFR) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7(4):201–7.
Das H, Abdulhameed N, Joseph M, Sakthivel R, Mao HQ, Pompili VJ. Ex vivo nanofiber expansion and genetic modification of human cord blood-derived progenitor/stem cells enhances vasculogenesis. Cell Transplant. 2009;18(3):305–18.
Das H, George JC, Joseph M, et al. Stem cell therapy with over expressed VEGF and PDGF genes improves cardiac function in a rat infarct model. PLoS ONE. 2009;4(10):e7325.
Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci. 2015;16:25476–501.
Duscher D, Barrera J, Wong VW, et al. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2016;62:216–25.
Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-base therapy: current status and perspectives. Cell Transplant. 2014;23:1045–59.
Quan R, Zheng X, Xu S, Zhang L, Yang D. Gelatin-chondroitin-6-sulfate-hyaluronic acid scaffold seeded with vascular endothelial growth factor 165 modified hair follicle stem cells as a three-dimensional skin substitute. Stem Cell Res Ther. 2014;5:118.
Bahrami H, Keshel SH, Chari AJ, Biazar E. Human unrestricted somatic stem cells loaded in nanofibrous PCL scaffold and their healing effect on skin defects. Artif Cells Nanomed Biotechnol. 2016;44:1556–60.
Kosaric N, Kiwanuka H, Gurtner GC. Stem cell therapies for wound healing. Expert Opin Biol Ther. 2019;19(6):575–85.
Cherbuin T, Movahednia MM, Toh WS, Cao T. Investigation of human embryonic stem cell-derived keratinocytes as an in vitro research model for mechanical stress dynamic response. Stem Cell Rev. 2015;11:460–73.
Wu DC, Boyd AS, Wood KJ. Embryonic stem cell transplantation: potential applicability in cell replacement therapy and regenerative medicine. Front Biosci. 2007;12:4525–35.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat Rev Mol Cell Biol. 2016;17:170–82.
Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
Leeb C, Jurga M, McGuckin C, Moriggl R, Kenner L. Promising new sources for pluripotent stem cells. Stem Cell Rev. 2010;6:15–26.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6.
Forraz N, McGuckin CP. The umbilical cord: a rich and ethical stem cell source to advance regenerative medicine. Cell Prolif. 2011;44:60–9.
Lin G, Xu RH. Progresses and challenges in optimization of human pluripotent stem cell culture. Curr Stem Cell Res Ther. 2010;5:207–14.
Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117.
Roh C, Lyle S. Cutaneous stem cells and wound healing. Pediatr Res. 2006;59:100R-103R.
Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 2010;19:491–502.
Alvarez-Dolado M, Martinez-Losa M. Cell fusion and tissue regeneration. Adv Exp Med Biol. 2011;713:161–75.
Yamanaka S, Blau HM. Nuclear reprogramming of a pluripotent state by three approaches. Nature. 2010;465(704):712.
Lluis F, Cosma MP. Cell-fusion-mediated somatic-cell reprogramming: a mechanism for tissue regeneration. J Cell Physiol. 2010;223:6–13.
Wagner J, Gluckman E. Umbilical cord blood transplantation: the first 20 years. Semin Hematol. 2010;47:3–12.
Kogler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–35.
Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in afibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–312.
Dawn B, Bolli R. Adult bone marrow-derived stem cells: regenerative potential, plasticity and tissue commitment. Basic Res Cardiol. 2005;100:495–503.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–8.
Rodda DJ, Chew JL, Lim LH, et al. Transcriptional regulation of nanog by oct4 and sox2. J Biol Chem. 2005;280:24731–7.
Zuk PA. The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21:1783–7.
Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60.
Wilson A, Butler PE, Seifalian AM. Adipose-derived stem cells for clinical applications: a review. Cell Prolif. 2011;44:86–98.
Utsunomiya T, Shimada M, Imura S, et al. Human adipose-derived stem cells: potential clinical applications in surgery. Surg Today. 2011;41:18–23.
Ogawa R. The importance of adipose-derived stem cells and vascularized tissue regeneration in the field of tissue transplantation. Curr Stem Cell Res Ther. 2006;1:13–20.
Sun N, Panetta NJ, Gupta DM, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci USA. 2009;106:15720–5.
Liu H, Chu Y, Lou G. Fiber-modified adenovirus can mediate human adipose tissue-derived mesenchymal stem cell-based anti-angiogenic gene therapy. Biotechnol Lett. 2010;32:181–1188.
Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: current findings and future perspectives. Discov Med. 2011;11:160–70.
Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25–33.
Hassanshahi A, Hassanshahi M, Khabbazi S, Hosseini-Khah Z, Peymanfar Y, Ghalamkari S, Su YW, Xian CJ. Adipose-derived stem cells for wound healing. J Cell Physiol. 2019;234(6):7903–14.
Knudtzon S. In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood. 1974;43:357–61.
McGuckin CP, Pearce D, Forraz N, Tooze JA, Watt SM, Pettengell R. Multiparametricanalysis of immature cell populations in umbilical cord blood and bone marrow. Eur J Haematol. 2003;71:341–50.
Denner L, Bodenburg Y, Zhao JG, et al. Directed engineering of umbilical cord blood stem cells to produce C-peptide and insulin. Cell Prolif. 2007;40:367–80.
Sobolewski K, Malkowski A, Barikowski E, Jaworski S. Wharton’s jelly as a reservoir of peptide growth factors. Placenta. 2005;26:747–52.
Majore I, Moretti P, Hass R, Kasper C. Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun Signal. 2009;7:6.
Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, Sugimoto M, Nakauchi H, Tojo A. Comparison of mesenchymal stem cells derived from arterial, venous and Wharton’s jelly explants of human umbilical cord. Int J Hematol. 2009;90:261–9.
Zhao Y, Wang H, Mazzone T. Identification of stem cells from human umbilical cord blood with embryonic and hematopoeitic characteristics. Exp Cell Res. 2006;312:2454–64.
Chunmeng S, Tianmin C. Skin: a promising reservoir for adult stem cell populations. Med Hypotheses. 2004;62:683–8.
Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102:5530–4.
Amoh Y, Katsuoka K, Hoffman RM. The advantages of hair follicle pluripotent stem cells over embryonic stem cells and induced pluripotent stem cells for regenerative medicine. J Dermatol Sci. 2010;60:131–7.
Amoh Y, Kanoh M, Niyama S, et al. Human hair follicle pluripotent stem (HFPS) cells promote regeneration of peripheral-nerve injury: an alternative to ES and iPS cells. J Cell Biochem. 2009;107:1016–20.
Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008;128:1311–8.
Witkowska-Zimny M, Walenko K. Stem cells from adipose tissue. Cell Mol Biol Lett. 2011;16:236–57.
Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806.
Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95.
Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–58.
Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–27.
Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multi lineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060–9.
De Bari C, Dell Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42.
Maumus M, Guerit D, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2:14.
Buhring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann NY Acad Sci. 2007;1106:262–71.
Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noel D. Immuno suppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1:2.
Krampera M, Cosmi L, Angeli R, Pasini, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398.
Frank MH, Sayegh MH. Immunomodulatory functions of mesenchymal stem cells. Lancet. 2004;363:1411–2.
Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–76.
Wang W, Li B, Yang J, et al. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibringel constructs. Biomaterials. 2010;31:8964–73.
Rama P, Matuska S, Paganoni G, Spinelli A, de Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–55.
Blocklet D, Toungouz M, Berkenboom G, et al. Myocardial homing of non mobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells. 2006;24:333–6.
Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–6.
Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27:6052–63.
Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr Rev. 2006;27:208–19.
Hunt DP, Morris PN, Sterling J, et al. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2008;26:163–72.
Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–8.
Erdo F, Buhrle C, Blunk J, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–5.
Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–41.
Sartipy P, Bjorquist P, Strehl R, Hyllner J. The application of human embryonic stem cell technologies to drug discovery. Drug Discov Today. 2007;12:688–99.
Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24.
Gorecka J, Gao X, Fereydooni A, Dash BC, Luo J, Lee SR, Taniguchi R, Hsia HC, Qyang Y, Dardik A. Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing. Regen Med. 2020;15(2):1277–93.
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41.
Ieda M, Fu JD, Delgado-Olquin P, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86.
Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–48.
Blanpain C. Stem cells: skin regeneration and repair. Nature. 2010;464:686–7.
Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63.
Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1358–66.
Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–17.
Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43.
Nijhof JG, Braun KM, Giangreco A, et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–37.
Jensen KB, Collins CA, Nascimento E, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–39.
Snippert HJ, Haegebarth A, Kasper M, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–9.
Williams DF. To engineer is to create: the link between engineering and regeneration. Trends Bio Technol. 2006;24:4–8.
Oshima H, Inoue H, Matsuzaki K, Tanabe M, Kumagai N. Permanent restoration of human skin treated with cultured epithelium grafting–wound healing by stem cell based tissue engineering. Hum Cell. 2002;15:118–28.
Christoph T, Muller-Rover S, Audring H, et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142:862–73.
Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial auto graft in the treatment of major burn injuries: a critical review of the literature. Burns. 2006;32:395–401.
Murata H, Janin A, Leboeuf C, et al. Donor-derived cells and human graft-versus-host disease of the skin. Blood. 2007;109(6):2663–5.
Jiang S, Walker L, Afentoulis M, et al. Transplanted human bone marrow contributes to vascular endothelium. Proc Natl Acad Sci USA. 2004;101(48):16891–6.
Kamolz LP, Kolbus A, Wick N, et al. Cultured human epithelium: human umbilical cord blood stem cells differentiate into keratinocytes under in vitro conditions. Burns. 2006;32(1):16–9.
Sackstein R. The bone marrow is akin to skin: HCELL and the biology of hematopoietic stem cell homing. J Invest Dermatol. 2004;122(5):1061–9.
Fu X, Sun X. Can hematopoietic stem cells be an alternative source for skin regeneration? Ageing Res Rev. 2009;8(2):44–249.
Kanji S, Das M, Aggarwal R, et al. Nanofiber-expanded human umbilical cord blood-derived CD34+ cell therapy accelerates cutaneous wound closure in NOD/SCID mice. J Cell Mol Med. 2014;18(4):685–97.
Hosseini MN. The role of keratinocyte function on the defected diabetic wound healing. Int J Burns Trauma. 2021;11(6):430–41.
Kim JY, Song SH, Kim KL, et al. Human cord blood derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010;19(12):1635–44.
Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35(8):1151–6.
Xu J, Wang D, Liu D, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood. 2012;120:3142–51.
Chen JS, Wong VW, Gurtner GC. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front Immunol. 2012;3:192.
Mathiasen AB, Jorgensen E, Qayyum AA, et al. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intra myocardial injection of autologous bone-marrow derived mesenchymal stromal cells in chronic ischemic heart failure (MSC-HF trial). Am Heart J. 2012;164:285–91.
Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86.
Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–6.
Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–7.
McFarlin K, Gao X, Liu YB, et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006;14:471–8.
Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell StemCell. 2009;5:54–63.
de Witte SFH, Luk F, Sierra Parraga JM, et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by Monocytic cells. Stem Cells. 2018;36:602–15.
Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–6.
Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806–15.
Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14(3):325–35.
Dong YX, Sigen A, Rodrigues M, et al. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv Func Mat. 2017;27:1606619.
Ho J, Walsh C, Yue D, Dardik A, Cheema U. Current advancements and strategies in tissue engineering for wound healing: a comprehensive review. Adv Wound Care. 2017;6:191–209.
Rustad KC, Wong VW, Sorkin M, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33:80–90.
Li Q, Zhang A, Tao C, Li X, Jin P. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2013;441:675–80.
Chen W, Li M, Yan ZL, et al. Effect of CXCR4 gene overexpression mediated by lentiviral vector on the biological characteristics of mesenchymal stem cells. Zhonghua Xue Ye Xue Za Zhi. 2013;34:440–4.
Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–99.
Thery C, Duban L, Segura E, et al. Indirect activation of naive CD4þ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–62.
Sarkar D, Ankrum JA, Teo GSL, Carman CV, Karp JM. Cellular and extracellular programming of cell fate through engineered intracrine-, paracrine-, and endocrine-like mechanisms. Biomaterials. 2011;32:3053–61.
Roger M, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31:8393–401.
Grisendi G, Spano C, D’souza N, et al. Mesenchymal progenitors expressing TRAIL induce apoptosis in sarcomas. Stem Cells. 2015;33:859–69.
Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–42.
Ranzani M, Cesana D, Bartholomae CC, et al. Lentiviral vector-based insertional mutagenesis identifies genes associated with liver cancer. Nat Methods. 2013;10:155–61.
Hinderer S, Layland SL, Schenke-Layland K. ECM and ECM-like materials-Biomaterials for applications in regenerative medicine and cancer therapy. Adv Drug Deliv Rev. 2016;97:260–9.
Stoppel WL, Ghezzi CE, McNamara SL, Black LD 3rd, Kaplan DL. Clinical applications of natural derived biopolymer-based scaffolds for regenerative medicine. Ann Biomed Eng. 2015;43:657–80.
Rabotyagova OS, Cebe P, Kaplan DL. Protein-based block copolymers. Biomacromol. 2011;12:269–89.
Ghasemi-Mobarakeh L, Prabhakaran MP, Tian L, Shamirzaei-Jeshvaghani E, Dehghani L, Ramakrishna S. Structural properties of scaffolds: crucial parameters towards stem cells differentiation. World J Stem Cells. 2015;7:728–44.
Willerth SM, Sakiyama-Elbert SE. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. Stem Book; Harvard Stem Cell Institute: Cambridge, MA, USA; 2008.
O’Loughlin A, Kulkarni M, Creane M, et al. Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes. 2013;62:2588–94.
Ozpur MA, Guneren E, Canter HI, et al. Generation of skin tissue using adipose tissue-derived stem cells. Plast Reconstr Surg. 2016;137:134–43.
Kim CH, Lee JH, Won JH, Cho MK. Mesenchymal stem cells improve wound healing in vivo via early activation of matrix metalloproteinase-9 and vascular endothelial growth factor. J Korean Med Sci. 2011;26:726–33.
Machula H, Ensley B, Kellar R. Electrospun tropoelastin for delivery of therapeutic adipose-derived stem cells to full-thickness dermal wounds. Adv Wound Care. 2014;3:367–75.
Almine JF, Bax DV, Mithieux SM, et al. Elastin-based materials. Chem Soc Rev. 2010;39:3371–9.
Navone SE, Pascucci L, Dossena M, et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res Ther. 2014;5:7.
Shen YI, Cho H, Papa AE, et al. Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials. 2016;102:107–19.
Bellini MZ, Caliari-Oliveira C, Mizukami A, et al. Combining xanthan and chitosan membranes to multipotent mesenchymal stromal cells as bioactive dressings for dermo-epidermal wounds. J Biomater Appl. 2015;29:1155–66.
Rodrigues C, de Assis AM, Moura DJ, et al. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS ONE. 2014;9:e96241.
Chen S, Shi J, Zhang M, et al. Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep. 2015;5:18104.
Leng M, Peng Y, Pan M, Wang H. Experimental study on the effect of allogeneic endothelial progenitor cells on wound healing in diabetic mice. J Diabetes Res. 2021;2021:9962877.
Baker MI, Walsh SP, Schwartz Z, Boyan BD. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J Biomed Mater Res Part B Appl Biomater. 2012;100:1451–7.
Ribeiro J, Pereira T, Amorim I, et al. Cell therapy with human MSCs isolated from the umbilical cord Wharton jelly associated to a PVA membrane in the treatment of chronic skin wounds. Int J Med Sci. 2014;11:979–87.
Gu J, Liu N, Yang X, Feng Z, Qi F. Adiposed-derived stem cells seeded on PLCL/P123 electrospun nanofibrous scaffold enhance wound healing. Biomed Mater. 2014;9:035012.
Geesala R, Bar N, Dhoke NR, Basak P, Das A. Porous polymer scaffold for on-site delivery of stem cells–protects from oxidative stress and potentiates wound tissue repair. Biomaterials. 2016;77:1–13.
Dong Y, Hassan WU, Kennedy R, et al. Performance of an insitu formed bioactive hydrogel dressing from a PEG-based hyperbranched multifunctional copolymer. Acta Biomater. 2014;10:2076–85.
Lee PY, Cobain E, Huard J, Huang L. Thermosensitive hydrogel PEG-PLGA-PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol Ther. 2007;15:1189–94.
Martino S, D’Angelo F, Armentano I, Kenny JM, Orlacchio A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol Adv. 2012;30:338–51.
Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–50.
Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36.
Sarasúa JG, Lopez SP, Viejo MA, et al. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J Spinal Cord Med. 2011;34:301–7.
Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359–66.
Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–77.
Garcia-Olmo D, Herreros D, De-La-Quintana P, et al. Adipose-derived stem cells in Crohn’s rectovaginal fistula. Case Rep Med. 2010;2010:961758.
Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422; discussion 1423–1424.
Lee HC, An SG, Lee HW, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76:1750–60.
Altman AM, Yan Y, Matthias N, et al. IFATS collection: human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27:250–8.
Nambu M, Kishimoto S, Nakamura S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009;62:317–21.
Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20:205–16.
Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24.
Maharlooei MK, Bagheri M, Solhjou Z, et al. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract. 2011;93:228–34.
Sivan-Loukianova E, Awad OA, Stepanovic V, Bickenbach J, Schatteman GC. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40(4):368–77.
Huang L, Wong YP, Gu H, et al. Stem cell-like properties of human umbilical cord lining epithelial cells and the potential for epidermal reconstitution. Cytotherapy. 2011;13:145–55.
Luo G, Cheng W, He W, et al. Promotion of cutaneous wound healing by local application of mesenchymal stem cells derived from human umbilical cord blood. Wound Repair Regen. 2010;18:506–13.
Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–8.
Akita S, Akino K, Hirano A, et al. Noncultured autologous adipose-derived stem cells therapy for chronic radiation injury. Stem Cells Int. 2010;2010:532704.
Akita S, Yoshimoto H, Ohtsuru A, et al. Autologous adipose-derived regenerative cells are effective for chronic intractable radiation injuries. Radiat Prot Dosimetry. 2012;151(4):656–60.
Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121(3):860–77.
Sarasua JG, Lopez SP, Viejo MA, et al. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J Spinal Cord Med. 2011;34(3):301–7.
Valbonesi M, Giannini G, Migliori F, et al. Cord blood (CB) stem cells for wound repair. Preliminary report of 2 cases. Transfus Apher Sci. 2004;30(2):153–6.
Zafarghandi MR, Ravari H, Aghdami N, et al. Safety and efficacy of granulocyte-colony-stimulating factor administration following autologous intramuscular implantation of bone marrow mononuclear cells: a randomized controlled trial in patients with advanced lower limb ischemia. Cytotherapy. 2010;12(6):783–91.
Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M, et al. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011;20(10):1629–39.
Sarasúa JG, López SP, Viejo MA, et al. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J Spinal Cord Med. 2011;34(3):301–7.
Kinoshita M, Fujita Y, Katayama M, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis. 2012;224(2):440–5.
Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27(11):2857–64.
Chen Y, Ma Y, Li N, et al. Efficacy and long-term longitudinal follow-up of bone marrow mesenchymal cell transplantation therapy in a diabetic patient with recurrent lower limb bullosis diabeticorum. Stem Cell Res Ther. 2018;9(1):99.
Lasala GP, Silva JA, Minguell JJ. Therapeutic angiogenesis in patients with severe limb ischemia by transplantation of a combination stem cell product. J Thorac Cardiovasc Surg. 2012;144(2):377–82.
Kirana S, Stratmann B, Prante C, et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract. 2012;66(4):384–93.
Prochazka V, Gumulec J, Jaluvka F, et al. Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant. 2010;19(11):1413–24.
Author information
Authors and Affiliations
Contributions
Dr. Ashish Garg and Dr. Sweta Garg compiled and wrote main manuscript text and Dr. Pradeep Adlak designed all figures and corrected all references as per journal guideline. Dr. Mohan Lal Kori and Dr. Santram Lodhi corrected the language of the entire manuscript to make its final form. All authors reviewed the manuscript before final submission.
Corresponding author
Ethics declarations
Conflict of Interest
The authors confirm that they have no conflict of interest to declare for this publication.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garg, A., Garg, S., Adlak, P. et al. Stem Cells and Regenerative Strategies for Wound Healing: Therapeutic and Clinical Implications. Curr. Pharmacol. Rep. 10, 121–144 (2024). https://doi.org/10.1007/s40495-024-00352-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-024-00352-4