Abstract
Purpose of Review
This article provides a brief overview of electrochemical potential-biological activity relationships of natural and synthetic cyclic sulfur-containing molecules against Steinernema feltiae, Botrytis cinerea, and Neuro 2a cell line (from murine neuroblastoma).
Recent Findings
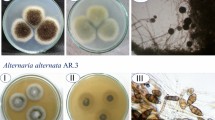
This article finds natural cyclic sulfur-containing molecules and their synthetic analogues were more reducing than glutathione (GSH) and therefore apparently did not react with GSH. The nematicidal assay indicated that cyclic disulfide compound of 1 (3-vinyl-4H-1,2-dithiin, 1,2-VDT) was more active against Steinernema feltiae with the LD50 value 151.93 ± 1.3 μM, while dithiole thione group compounds showed moderate activity against this nematode. The article also finds compound 7 (3H-1,2-dithiole-3-thione or dithiolethione, DT) has a strong activity against all different strains of Botrytis cinerea in the range concentration of 0.1–0.5 mM. This article also finds that compounds 3 (1,2-dithiane, 1,2-DT), 4 (1,5-dithiacyclooctane, 1,5-DTCO), and 7 (3H-1,2-dithiole-3-thione or dithiolethione, DT) possess some moderate activity on Neuro 2a cell lines.
Summary
Antinematode, antifungal, and anticancer activity of cyclic sulfur-containing molecules indicated that they could be promising candidates for “green pesticides” or phytoprotectans and for cancer prevention.









Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Bio Med. 2001;30:1191–212.
Jacob C, Giles GL, Giles NM, Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed. 2003;42:4742–58.
Giles GI, Tasker KM, Jacob C. Hypothesis: the role of reactive sulfur species in oxidative stress. Free Radical Bio Med. 2001;31:1279–83.
Doering M. Development of multifunctional agents and their biological evaluation in the context of cancer and inflammatory diseases. Department of Bioorganic Chemistry. School of Pharmacy. Universitaet des Saarlandes. Dissertation. 2012.
Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11.
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84.
Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7:200–3.
Czepukojc B, Viswanathan UM, Raza A, Ali S, Burkholz T, Jacob C. Tetrasulfanes as selective modulators of the cellular thiolstat. Phosphorus Sulfur. 2013;188:446–53.
Jacob C, Battaglia E, Burkholz T, Peng D, Bagrel D, Montenarh M. Control of oxidative posttranslational cysteine modifications: from intricate chemistry to widespread biological and medical applications. Chem Res Toxicol. 2012;25:588–604.
Jacob C, Ba LA. Open season for hunting and trapping post-translational cysteine modifications in proteins and enzymes. Chembiochem. 2011;12(6):841–4.
•• Jacob C. Redox signalling via the cellular thiolstat. Biochem Soc Trans. 2011;39:1247–53. This paper summarizes ‘thiolstat’ is provided by the complex redox chemistry of the amino acid cysteine, able to participate in different redox processes.
Weber ND, Andersen DO, North JA, Murray BK, Lawson LD, Hughes BG. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58:417–23.
Block E. Garlic and other alliums—the lore and the science. Cambridge: Royal Society of Chemistry; 2010.
Shams-Ghahfarokhi M, Shokoohamiri MR, Amirrajab N, Moghadasi B, Ghajari A, Zeini F, et al. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia. 2006;77:321–3.
• Jacob C. A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms. Nat Prod Rep. 2006;23:851–63. This paper summarizes that sulfur-containing molecules allow reactive sulfur species (RSS) to interact with oxidative stressors, to affect the function of redox-sensitive cysteine proteins and to disrupt the integrity of DNA and cellular membranes.
Block E, Ahmad S, Catalfamo JL, Jain MK, Apitzcastro R. Antithrombotic organosulfur compounds from garlic - structural, mechanistic, and synthetic studies. J Am Chem Soc. 1986;108:7045–55.
Okada Y, Tanaka K, Fujita I, Sato E, Okajima H. Antioxidant activity of thiosulfinates derived from garlic. Redox Rep. 2005;10:96–102.
Egenschwind C, Eckard R, Jekat FW, Winterhoff H. Pharmacokinetics of Vinyldithiins, Transformation products of allicin. Planta Med. 1992;58:8–13.
Zhou S-F. Supportive cancer care with Chinese medicine. Netherlands: Springer; 2010.
Beslin P. A facile synthesis of 2 thioacrolein dimers - a new entry to a flavor component in asparagus. J Heterocyclic Chem. 1983;20:1753–4.
Manabe T, Hasumi A, Sugiyama M, Yamazaki M, Saito K. Alliinase [S-alk(en)yl-L-cysteine sulfoxide lyase] from Allium tuberosum (Chinese chive) - purification, localization, cDNA cloning and heterologous functional expression. Eur J Biochem. 1998;257:21–30.
Flora SJ. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev. 2009;2:191–206.
Sendl A, Schliack M, Loser R, Stanislaus F, Wagner H. Inhibition of cholesterol-synthesis in vitro by extracts and isolated compounds prepared from garlic and wild garlic. Atherosclerosis. 1992;94:79–85.
Sheu CM, Foote CS, Gu CL. Photooxidation of 1,5-dithiacyclooctane - a novel C-S bond-cleavage. J Am Chem Soc. 1992;114:3015–21.
Tazzari V, Cappelletti G, Casagrande M, Perrino E, Renzi L, Del Soldato P, et al. New aryldithiolethione derivatives as potent histone deacetylase inhibitors. Bioorg Med Chem. 2010;18:4187–94.
Curphey TJ. Thionation with the reagent combination of phosphorus pentasulfide and hexamethyldisiloxane. J Organomet Chem. 2002;67:6461–73.
Munday R, Zhang Y, Paonessa JD, Munday CM, Wilkins AL, Babu J. Synthesis, biological evaluation, and structure-activity relationships of dithiolethiones as inducers of cytoprotective phase 2 enzymes. J Med Chem. 2010;53:4761–7.
Munday R, Munday CM. Induction of phase II enzymes by 3H-1,2-dithiole-3-thione: dose-response study in rats. Carcinogenesis. 2004;25(9):1721–5.
Hamann CH, Vielstich W. Elektrochemie. Weinheim: Wiley VCH Verlag GmbH; 2005.
Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. 2nd ed. New York: A John Wiley & Sons; 2001.
Brett CMA, Oliveira Brett AM. Electrochemistry, Principles, methods, and applications. Oxford: Oxford Science Publications; 1993.
Hamann CH, Vielstich W. Chemie Ingenieur Technik. Weinheim: Wiley VCH Verlag GmbH; 1999.
Kissinger PT, Heineman WR. Cyclic voltammetry. J Chem Educ. 1983;60:702–6.
Stenken JA, Puckett DL, Lunte SM, Lunte CE. Detection of N-acetylcysteine, cysteine and their disulfides in urine by liquid chromatography with a dualelectrode amperometric detector. J Pharm Biomed Anal. 1990;8:85–9.
Adam V, Fabrik I, Kohoutkova V, et al. Automated electrochemical analyzer as a new tool for detection of thiols. Int J Electrochem Sci. 2010;5:429–47.
Ralph TR, Hitchman ML, Millington JP, Walsh FC. The electrochemistry of L-cystine and L-cysteine.1. Thermodynamic and kinetic-studies. J Electroanal Chem. 1994;375:1–15.
• Jacob C, Special issue: redox active natural products and their interaction with cellular signalling pathways. Molecules. 2014;19:19588–93. This paper summarizes that redox active compounds, especially of reactive sulfur species (RSS) is a powerful platform to "intracellular diagnostics" in different organisms.
•• Domínguez Álvarez E, Viswanathan UM, Burkholz T, Khairan K, Jacob C. Bio-Electrochemistry and Chalcogens. Chapter 7 in M. Schlesinger (ed.). Applications of Electrochemistry in Medicine. Modern Aspects of Electrochemistry. 2013; 56:249-282. This paper summarizes that redox active compounds, especially of reactive sulfur species (RSS) is a powerful platform to "intracellular diagnostics" in different organisms.
• Burkholz T. Oxidative stress and electrochemical procedure for surface decontamination in dialyses. Department of Bioorganic Chemistry. School of Pharmacy. Universitaet des Saarlandes. Dissertation. 2010. This paper summarizes that cyclic voltammetry is able to monitor (bio-) chemical process or redox processes at the cellular level.
Sarakbi MB. Natural Products and related compounds as promising antioxidants and antimicrobial agents. Department of Bioorganic Chemistry. School of Pharmacy. Universitaet des Saarlandes. Diplom. 2009.
Clennan EL, Liao C. The hydroperoxysulfonium ylide. An aberration or a ubiquitous intermediate? Tetrahedron. 2006;62:10724–8.
Buecher EJ, Popiel I. Liquid culture of the entomogenous nematode Steinernema feltiae with its bacterial symbiont. J Nematol. 1989;21:500–4.
Collado IG, Sanchez AJ, Hanson JR. Fungal terpene metabolites: biosynthetic relationships and the control of the phytopathogenic fungus Botrytis cinerea. Nat Prod Rep. 2007;24:674–86.
Calderon FH, Bonnefont A, Munoz FJ, Fernandez V, Videla LA, Inestrosa NC. PC12 and neuro 2a cells have different susceptibilities to acetylcholinesterase-amyloid complexes, amyloid 25-35 fragment, glutamate, and hydrogen peroxide. J Neurosci Res. 1999;56:620–31.
Kaneshiro ES, Wyder MA, Wu YP, Cushion MT. Reliability of calcein acetoxy methyl-ester and ethidium homodimer or propidium iodide for viability assessment of microbes. J Microbiol Methods. 1993;17:1–16.
Whittemore ER, Loo DT, Watt JA, Cotman CW. A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neuroscience. 1995;67:921–32.
Whittemore ER, Loo DT, Cotman CW. Exposure to hydrogen peroxide induces cell death via apoptosis in cultured rat cortical neurons. Neuroreport. 1994;5:1485–8.
Schneider T, Muthukumar Y, Hinkelmann B, Franke R, Doring M, Jacob C, et al. Deciphering intracellular targets of organochalcogen based redox catalysts. Medchemcomm. 2012;3:784–7.
Doering M, Diesel B, Gruhlke MCH, Viswanathan UM, Manikova D, Chovanec M, et al. Selenium- and tellurium-containing redox modulators with distinct activity against macrophages: possible implications for the treatment of inflammatory diseases. Tetrahedron. 2012;68:10577–85.
Spencer JPE, Jenner A, Aruoma OI, Evans PJ, Kaur H, Dexter DT, et al. Intense oxidative DNA-damage promoted by L-dopa and its metabolites implications for neurodegenerative disease. FEBS Lett. 1994;353:246–50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International Lisence (http://creativecommons.org/licences /by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons lisences, and indicate if changes were made.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Redox Modulators
Rights and permissions
About this article
Cite this article
Khairan, K., Ba, L.A., Burkholz, T. et al. Electrochemical Potential-Biological Activity Relationships of Cyclic Sulfur-Containing Molecules Against Steinernema feltiae, Botrytis cinerea, and Neuro 2a Cell Line. Curr Pharmacol Rep 5, 174–187 (2019). https://doi.org/10.1007/s40495-019-00179-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-019-00179-4