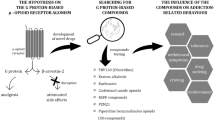
Abstract
Purpose of Review
Opioids have a long history of use for relief of pain dating back thousands of years. Opium derived from the poppy plant is one of the earliest substances used for pain relief and continues to be employed as potent therapeutics for both acute and chronic pain. Despite the rapid and potent effects of opioids, their extensive negative side effect profile limits their clinical use. Some of the side effects associated with opioid use include constipation, nausea and vomiting, drowsiness and sedation, respiratory depression, itching, dizziness, and the development of opioid tolerance and dependence with prolonged use. Studies have revealed that the formation of dependence and tolerance involves not only a single intracellular signaling pathway but rather a complex communication network. While there is still an incomplete understanding of the mechanisms behind opioid tolerance and dependence, research has advanced our understanding, particularly focusing on the intracellular activation and regulation of opioid receptors. This article aims to unravel the complexities of opioid receptor function as we delve into the intricate network of intracellular signaling pathways and receptor regulation while offering insights into innovative methods that have emerged to address the potential side effects of opioid use.
Recent Findings
Opioids stimulate the release of dopamine primarily via the μ-opioid receptor (MOR) in the brain's reward pathway. This activation reinforces drug-seeking behavior and contributes to the development of addiction. In recent years, efforts have been made to intervene in the complex network of molecular-level opioid signaling, aiming to preserve the analgesic effects while reducing the serious side effects associated with opioid use. Some interventions focus on RGS proteins which play a crucial role in opioid signaling, while others explore the distinct effects created in intracellular G protein signaling using "biased" agonists. Another idea involves manipulating κ-opioid receptors (KORs) in the brain's reward pathway, which exhibit opposing effects to MORs. This approach aims not only to understand opioid addiction but also to shed light on conditions such as alcohol, drug, and food addiction.
Summary
This review article addresses molecular studies that have been focused on in recent years, especially against opioid dependence and other side effects, and it has raised the question of whether the side effects of opioids can be eliminated in the future. These studies not only contribute to the efforts to prevent addiction and other side effects of opioids but also to the elucidation of the intracellular signaling network in opioid receptor activation. This complex signaling network is becoming better understood with each passing day, contributing to the improved manipulation of opioid signaling.
Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Zieglgänsberger W. Substance P and Pain Chronicity. Cell Tissue Res. 2019;375:227–41. https://doi.org/10.1007/s00441-018-2922-y.
Cansız D, Emekli-Alturfan E, Ata Alturfan A. Effects of endogenous opioids on pain mechanism. Experimed. 2021;11:49–56. https://doi.org/10.26650/experimed.2021.884254.
Stein C. Opioid analgesia: recent developments. Curr Opin Support Palliat Care. 2020;14:112–7. https://doi.org/10.1097/SPC.0000000000000495.
Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol. 2009;9:3–8. https://doi.org/10.1016/j.coph.2008.12.009.
Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–81. https://doi.org/10.1097/ALN.0b013e318238bba6.
Mercadante S, Arcuri E, Santoni A. Opioid-induced tolerance and hyperalgesia. CNS Drugs. 2019;33:943–55. https://doi.org/10.1007/s40263-019-00660-0.
Che T, Roth BL. Molecular basis of opioid receptor signaling. Cell. 2023;186:5203–19. https://doi.org/10.1016/j.cell.2023.10.029.
Gopalakrishnan L, Chatterjee O, Ravishankar N, Suresh S, Raju R, Mahadevan A, Prasad TSK. Opioid receptors signaling network. J Cell Commun Signal. 2022;16:475–83. https://doi.org/10.1007/s12079-021-00653-z.
Jordan BA, Cvejic S, Devi LA. Opioids and their complicated receptor complexes. Neuropsychopharmacol. 2000;23:S5–18. https://doi.org/10.1016/S0893-133X(00)00143-3.
Syrovatkina V, Alegre KO, Dey R, Huang XY. Regulation, signaling, and physiological functions of G-proteins. J Mol Biol. 2016;428:3850–68. https://doi.org/10.1016/j.jmb.2016.08.002.
Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. https://doi.org/10.1152/physrev.2001.81.1.299.
Halls ML, Cooper DMF. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol. 2011;3:a004143. https://doi.org/10.1101/cshperspect.a004143.
Ostrom KF, Lavigne JE, Brust TF, Seifert R, Dessauer CW, Watts VJ, Ostrom RS. Physiological roles of mammalian transmembrane adenylyl cyclase isoforms. Physiol Rev. 2022;102:815–57. https://doi.org/10.1152/physrev.00013.2021.
Svoboda KR, Lupica CR. Opioid inhibition of hipposvoboda KR, Lupica CR. opioid inhibition of hippocampal interneurons via modulation of potassium and hyperpolarization-activated cation (Ih) currents. J Neurosci. 1998;18:7084–98. https://doi.org/10.1523/JNEUROSCI.18-18-07084.1998.
Watts VJ, Neve KA. Sensitization of adenylate cyclase by Gαi/o-coupled receptors. Pharmacol Ther. 2005;106:405–21. https://doi.org/10.1016/j.pharmthera.2004.12.005.
Khan SM, Sung JY, Hébert TE. Gβγ Subunits—different spaces, different faces. Pharmacol Res. 2016;111:434–41. https://doi.org/10.1016/j.phrs.2016.06.026.
Bian JM, Wu N, Su RB, Li J. Opioid receptor trafficking and signaling: what happens after opioid receptor activation? Cell Mol Neurobiol. 2012;32:167–84. https://doi.org/10.1007/s10571-011-9755-5.
Mathews JL, Smrcka AV, Bidlack JM. A novel Gβγ-subunit inhibitor selectively modulates μ-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J Neurosci. 2008;28:12183–9. https://doi.org/10.1523/JNEUROSCI.2326-08.2008.
Gintzler AR, Chakrabarti S. Post-opioid receptor adaptations to chronic morphine; altered functionality and associations of signaling molecules. Life Sci. 2006;79:717–22. https://doi.org/10.1016/j.lfs.2006.02.016.
Paul AK, Smith CM, Rahmatullah M, Nissapatorn V, Wilairatana P, Spetea M, Gueven N, Dietis N. Opioid analgesia and opioid-induced adverse effects: a review. Pharmaceuticals. 2021;14:1091. https://doi.org/10.3390/ph14111091.
Karkhanis A, Holleran KM, Jones SR. Dynorphin/kappa opioid receptor signaling in preclinical models of alcohol, drug, and food addiction. Int Rev Neurobiol. 2017;136:53–88. https://doi.org/10.1016/bs.irn.2017.08.001.
Bloodgood DW, Hardaway JA, Stanhope CM, Pati D, Pina MM, Neira S, Desai S, Boyt KM, Palmiter RD, Kash TL. Kappa opioid receptor and dynorphin signaling in the central amygdala regulates alcohol intake. Mol Psychiatry. 2021;26:2187–99. https://doi.org/10.1038/s41380-020-0690-z.
Bailey C, Oldfield S, Llorente J, Caunt C, Teschemacher A, Roberts L, et al. Involvement of PKCα and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of µ-opioid receptors in mature brain neurons. Br J Pharmacol. 2009;158:157–64. https://doi.org/10.1111/j.1476-5381.2009.00140.x.
Bailey CP, Llorente J, Gabra BH, Smith FL, Dewey WL, Kelly E, et al. Role of protein kinase C and μ-opioid receptor (MOPr) desensitization in tolerance to morphine in rat locus coeruleus neurons. Eur J Neurosci. 2009;29:307–18. https://doi.org/10.1111/j.1460-9568.2008.06573.x.
Smith F, Javed R, Smith P, Dewey W, Gabra B. PKC and PKA inhibitors reinstate morphine-induced behaviors in morphine tolerant mice. Pharmacol Res. 2006;54:474–80. https://doi.org/10.1016/j.phrs.2006.09.007.
Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, et al. Agonist-selective mechanisms of μ-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70:676–85. https://doi.org/10.1124/mol.106.022376.
Zuo Z. The role of opioid receptor internalization and ??-arrestins in the development of opioid tolerance. Anesth Analg. 2005;101:728–34. https://doi.org/10.1213/01.ANE.0000160588.32007.AD.
Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Regulation of µ -opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–54. https://doi.org/10.1152/physrev.2001.81.1.299.
Zhang JJ, Song CG, Wang M, Zhang GQ, Wang B, Chen X, Lin P, Zhu YM, Sun ZC, Wang YZ, Jiang JL, Li L, Yang XM, Chen ZN. Monoclonal antibody targeting mu-opioid receptor attenuates morphine tolerance via enhancing morphine-induced receptor endocytosis. J Pharm Anal. 2023;13:1135–52; https://doi.org/10.1016/j.jpha.2023.06.008. The importance of this article is that; manipulations in the Mu-opioid receptor signaling have been made by using monoclonal antibodies, and more importantly, it has contributed to elucidating the intracellular signaling pathways in morphine-dependent signaling.
Eichel K, von Zastrow M. Subcellular organization of GPCR signaling. Trends Pharmacol Sci. 2018;39:200–8. https://doi.org/10.1016/j.tips.2017.11.009.
Thomsen ARB, Jensen DD, Hicks GA, Bunnett NW. Therapeutic targeting of endosomal G-protein-coupled receptors. Trends Pharmacol Sci. 2018;39:879–91. https://doi.org/10.1016/j.tips.2018.08.003.
Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol. 2014;27:109–16. https://doi.org/10.1016/j.ceb.2013.10.003.
Lobingier BT, von Zastrow M. When trafficking and signaling mix: How subcellular location shapes G protein-coupled receptor activation of heterotrimeric G proteins. Traffic. 2019;20:130–6. https://doi.org/10.1111/tra.12634.
Jimenez-Vargas NN, Gong J, Wisdom MJ, Jensen DD, Latorre R, Hegron A, et al. Endosomal signaling of delta opioid receptors is an endogenous mechanism and therapeutic target for relief from inflammatory pain. Proc Natl Acad Sci USA. 2020;117:15281–92. https://doi.org/10.1073/pnas.2000500117.
Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, et al. A nontoxic pain killer designed by modeling of pathological receptor conformations. Science. 2017;355:966–9. https://doi.org/10.1126/science.aai8636.
Chan P, Lutfy K. Molecular changes in opioid addiction: the role of adenylyl cyclase and CAMP/PKA system. Prog Mol Biol Transl Sci. 2016;137:203–27. https://doi.org/10.1016/bs.pmbts.2015.10.005.
Llorca-Torralba M, Pilar-Cuéllar F, Bravo L, Bruzos-Cidon C, Torrecilla M, Mico JA, Ugedo L, Garro-Martínez E, Berrocoso E. Opioid activity in the locus coeruleus is modulated by chronic neuropathic pain. Mol Neurobiol. 2019;56:4135–50. https://doi.org/10.1007/s12035-018-1361-9.
Çağlıyan H, Solak Görmüş I, Özen Koca R. Determination of physiological changes in the heart in rats with an experimental morphine addiction model. [Master's thesis, Necmettin Erbakan University, Department of Physiology]. 2021.
Listos J, Łupina M, Talarek S, Mazur A, Orzelska-Górka J, Kotlińska J. The mechanisms involved in morphine addiction: an overview. Int J Mol Sci. 2019;20:4302. https://doi.org/10.3390/ijms20174302.
Pirino BE, Kelley AM, Karkhanis AN, Barson JR. A critical review of effects on ethanol intake of the dynorphin/kappa opioid receptor system in the extended amygdala: from inhibition to stimulation. Alcohol Clin Exp Res. 2023;47:1027–38. https://doi.org/10.1111/acer.15078.
Margolis EB, Wallace TL, Van Orden LJ, Martin WJ. Differential effects of novel kappa opioid receptor antagonists on dopamine neurons using acute brain slice electrophysiology. PLoS One. 2020;15:e0232864. https://doi.org/10.1371/journal.pone.0232864.
Crowley NA, Kash TL. Kappa opioid receptor signaling in the brain: circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2015;62:51–60. https://doi.org/10.1016/j.pnpbp.2015.01.001.
Bruijnzeel AW. Kappa-opioid receptor signaling and brain reward function. Brain Res Rev. 2009;62:127–46. https://doi.org/10.1016/j.brainresrev.2009.09.008.
Zan GY, Wang Q, Wang YJ, Liu Y, Hang A, Shu XH, Liu JG. Antagonism of κ opioid receptor in the nucleus accumbens prevents the depressive-like behaviors following prolonged morphine abstinence. Behav Brain Res. 2015;291:334–41. https://doi.org/10.1016/j.bbr.2015.05.053.
Erikson CM, Wei G, Walker BM. Maladaptive behavioral regulation in alcohol dependence: role of kappa-opioid receptors in the bed nucleus of the stria terminalis. Neuropharmacology. 2018;140:162–73. https://doi.org/10.1016/j.neuropharm.2018.07.034.
Senese NB, Kandasamy R, Kochan KE, Traynor JR. Regulator of G-Protein Signaling (RGS) protein modulation of opioid receptor signaling as a potential target for pain management. Front Mol Neurosci. 2020;13:5. https://doi.org/10.3389/fnmol.2020.00005.
Hooks SB, Martemyanov K, Zachariou V. A role of RGS proteins in drug addiction. Biochem Pharmacol. 2008;75:76–84. https://doi.org/10.1016/j.bcp.2007.07.045.
Sutton LP, Ostrovskaya O, Dao M, Xie K, Orlandi C, Smith R, Wee S, Martemyanov KA. Regulator of G-protein signaling 7 regulates reward behavior by controlling opioid signaling in the striatum. Biol Psychiatry. 2016;80:235–45. https://doi.org/10.1016/j.biopsych.2015.07.026.
Garzón J, López-Fando A, Sánchez-Blázquez P. The R7 subfamily of RGS proteins assists tachyphylaxis and acute tolerance at μ-opioid receptors. Neuropsychopharmacology. 2003;28:1983–90. https://doi.org/10.1038/sj.npp.1300263.
Papachatzaki MM, Antal Z, Terzi D, Szücs P, Zachariou V, Antal M. RGS9–2 modulates nociceptive behaviour and opioid-mediated synaptic transmission in the spinal dorsal horn. Neurosci Lett. 2011;501:31–4. https://doi.org/10.1016/j.neulet.2011.06.033.
Sakloth F, Polizu C, Bertherat F, Zachariou V. Regulators of G protein signaling in analgesia and addiction. Mol Pharmacol. 2020;98:739–50. https://doi.org/10.1124/MOL.119.119206.
Morgan MM, Tran A, Wescom RL, Bobeck EN. Differences in antinociceptive signalling mechanisms following morphine and fentanyl microinjections into the rat periaqueductal gray. Eur J Pain. 2020;24:617–24. https://doi.org/10.1002/ejp.1513.
Bosier B, Doyen PJ, Brolet A, Muccioli GG, Ahmed E, Desmet N, Hermans E, Deumens R. Inhibition of the regulator of g protein signalling RGS4 in the spinal cord decreases neuropathic hyperalgesia and restores cannabinoid CB1 receptor signalling. Br J Pharmacol. 2015;172:5333–46. https://doi.org/10.1111/bph.13324.
Kaski SW, White AN, Gross JD, Siderovski DP. Potential for kappa-opioid receptor agonists to engineer nonaddictive analgesics: a narrative review. Anesth Analg. 2021;132:406–19. https://doi.org/10.1213/ANE.0000000000005309.
Faouzi A, Varga BR, Majumdar S. Biased opioid ligands. Molecules. 2020;25:4257. https://doi.org/10.3390/molecules25184257.
El Daibani A, Paggi JM, Kim K, Laloudakis YD, Popov P, Bernhard SM, Krumm BE, Olsen RHJ, Diberto J, Carroll FI, Katrich V, Wünsch B, Dror RO, Che T. Molecular mechanism of biased signaling at the kappa opioid receptor. Nat Commun. 2023;14: https://doi.org/10.1038/s41467-023-37041-7
Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, Henderson G, Christie MJ, Schulz S. Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br J Pharmacol. 2020;177:2923–31. https://doi.org/10.1111/bph.15004.
Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aubé J, Jones SR, Martin TJ, Bohn LM. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal. 2016;9:ra117. https://doi.org/10.1126/scisignal.aai8441.
Huskinson SL, Platt DM, Zamarripa CA, Dunaway K, Brasfield M, Prisinzano TE, Blough BE, Freeman KB. The G-protein biased kappa opioid agonists, triazole 1.1 and nalfurafine, produce non-uniform behavioral effects in male rhesus monkeys. Pharmacol Biochem Behav. 2022;217; https://doi.org/10.1016/j.pbb.2022.173394. This article is important since it points out that using biased KOR agonists could be potential potent analgesics without the serious side effects caused by classical KOR agonists.
Huskinson SL, Platt DM, Brasfield M, Follett ME, Prisinzano TE, Blough BE, Freeman KB. Quantification of observable behaviors induced by typical and atypical kappa-opioid receptor agonists in male rhesus monkeys. Psychopharmacology (Berl). 2020;237:2075–87. https://doi.org/10.1007/s00213-020-05519-7.
Groom S, Blum NK, Conibear AE, Disney A, Hill R, Husbands SM, Li Y, Toll L, Kliewer A, Schulz S, Henderson G, Kelly E, Bailey CP. A novel G protein-biased agonist at the μ opioid receptor induces substantial receptor desensitisation through G protein-coupled receptor kinase. Br J Pharmacol. 2023;180:943–57; https://doi.org/10.1111/bph.15334. In light of the information presented in this article, it has been determined that contrary to previous suggestions, not all G protein-biased agonists show positive effects in receptor desensitization; in fact, they can exhibit the opposite effects.
Funding
No specific fundings have been received for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.B.B., R.O.K; literature search and data analysis: M.B.B.; writing—original draft preparation: M.B.B.; writing-review and editing: R.O.K. and Z.I.S.G. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Activation of dynorphin/kappa-opioid receptor system induces dysphoric effects, which may contribute to substance addiction with a negative reinforcement.
• Inhibition of some regulators of G-protein signaling (RGS) proteins has some promising effects on enhancing the analgesic activity and reducing the side effects of opioids.
• Side effects occurring through β-arrestin2 pathway may be eliminated by using G-protein biased opioid receptor agonists.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Basaran, M.B., Koca, R.O. & Gormus, Z.I.S. Therapeutic Innovations Against Opioid Tolerance and Addiction. Curr Behav Neurosci Rep (2024). https://doi.org/10.1007/s40473-024-00277-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s40473-024-00277-8