Abstract
Purpose
This study explored the feasibility of 68gallium (Ga)-labeled novel fibroblast activation protein (FAP)-targeted ligand for tumor imaging through small-animal PET/CT (positron emission computed tomography/computed tomography).
Methods
The FAP-ligand (FL) was created by adding the chelating group dodecane tetraacetic acid (DOTA) and labeling with 68Ga. The MC38 cell line was used to establish a C57BL/6 mice colon cancer model. The radioactivity distribution of labeled 68Ga-DOTA-FL across various organs of the mouse model was examined ex-vivo. In addition, 68Ga-DOTA-FL tumor-targeted imaging in vivo was performed using small-animal PET/CT. Finally, western blotting and immunofluorescence imaging of MC38 cells and xenotransplant tumor tissues were conducted.
Results
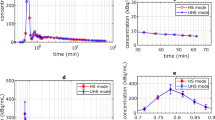
The radiolabeling rate and radiochemical purity of 68Ga-DOTA-FL were above 95%. Both western blotting and immunofluorescence imaging revealed FAP expression in the tumor tissues, but not in the MC38 cells. Small-animal PET/CT imaging indicated that the tumor imaging was clearest at 30 min after 68Ga-DOTA-FL treatment. Examination of the radioactivity distribution in vitro revealed that at 30 min after the 68Ga-DOTA-FL treatment, the target/non-target ratio for tumor and muscle tissue was 4.0 ± 0.3 (n = 3).
Conclusions
68Ga-DOTA-FL can be used for the specific tumor imaging in mouse models, which might provide a novel alternative for FAP-targeted tumor imaging.





Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Roy J, Hettiarachchi SU, Kaake M, Mukkamala R, Low PS (2020) Design and validation of fibroblast activation protein alpha targeted imaging and therapeutic agents. Theranostics 10(13):5778–5789
Scott AM, Wiseman G, Welt S et al (2003) A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res 9(5):1639–1647
Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H (2015) Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel) 7(4):2443–2458
Tao L, Huang G, Song H, Chen Y, Chen L (2017) Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett 14(3):2611–2620
Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC (2016) Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res 18(1):84
Ziani L, Chouaib S, Thiery J (2018) Alteration of the antitumor immune response by cancer-associated fibroblasts. Front Immunol 9:414
Barbazan J, Matic VD (2019) Cancer associated fibroblasts: is the force the path to the dark side? Curr Opin Cell Biol 56:71–79
Lo PC, Chen J, Stefflova K et al (2009) Photodynamic molecular beacon triggered by fibroblast activation protein on cancer-associated fibroblasts for diagnosis and treatment of epithelial cancers. J Med Chem 52(2):358–368
Brennen WN, Isaacs JT, Denmeade SR (2012) Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther 11(2):257–266
Li J, Chen K, Liu H et al (2012) Activatable near-infrared fluorescent probe for in vivo imaging of fibroblast activation protein-alpha. Bioconjug Chem 23(8):1704–1711
Pandya DN, Sinha A, Yuan H et al (2020) Imaging of fibroblast activation protein alpha expression in a preclinical mouse model of glioma using positron emission tomography. Molecules. https://doi.org/10.3390/molecules25163672
Calais J (2020) FAP: the next billion dollar nuclear theranostics target? J Nucl Med 61(2):163–165
Altmann A, Haberkorn U, Siveke J (2021) The latest developments in imaging of fibroblast activation protein. J Nucl Med 62(2):160–167
Hathi DK, Jones EF (2019) (68)Ga FAPI PET/CT: tracer uptake in 28 different kinds of cancer. Radiol Imaging Cancer 1(1):e194003
Backhaus P, Gierse F, Burg MC et al (2022) Translational imaging of the fibroblast activation protein (FAP) using the new ligand [(68)Ga]Ga-OncoFAP-DOTAGA. Eur J Nucl Med Mol Imaging 49(6):1822–1832
Giesel FL, Kratochwil C, Lindner T et al (2019) (68)Ga-FAPI PET/CT: Biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med 60(3):386–392
Lindner T, Loktev A, Altmann A et al (2018) Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med 59(9):1415–1422
Meyer C, Lindner MT et al (2020) Radiation dosimetry and biodistribution of (68)Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med 61(8):1171–1177
Baum RP, Schuchardt C, Singh A et al (2022) Feasibility, biodistribution, and preliminary dosimetry in peptide-targeted radionuclide therapy of diverse adenocarcinomas using (177)Lu-FAP-2286: first-in-humans results. J Nucl Med 63(3):415–423
Liu Y, Watabe T, Kaneda-Nakashima K et al (2022) Fibroblast activation protein targeted therapy using [(177)Lu]FAPI-46 compared with [(225)Ac]FAPI-46 in a pancreatic cancer model. Eur J Nucl Med Mol Imaging 49(3):871–880
Kuyumcu S, Kovan B, Sanli Y et al (2021) Safety of fibroblast activation protein-targeted radionuclide therapy by a low-dose dosimetric approach using 177Lu-FAPI04. Clin Nucl Med 46(8):641–646
Fu K, Pang Y, Zhao L et al (2022) FAP-targeted radionuclide therapy with [(177)Lu]Lu-FAPI-46 in metastatic nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging 49(5):1767–1769
Fu H, Huang J, Sun L, Wu H, Chen H (2022) FAP-targeted radionuclide therapy of advanced radioiodine-refractory differentiated thyroid cancer with multiple cycles of 177Lu-FAPI-46. Clin Nucl Med 47(10):906–907
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant number 82001863) and the Shanghai Sailing Program (grant number 19YF1408200). We are grateful to the staff of the Department of Nuclear Medicine of Ruijin Hospital Affiliated to Shanghai Jiaotong University and to Wangxi Hai; Yang Liu and Yifan Zhang who contributed to the small-animal PET/CT imaging work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ZX, YS, HY and JL. The first draft of the manuscript was written by JL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors (Zhan Xu, Yimeng Shi, Hongyan Yin, and Jing Lv) declare that they have no competing interests.
Ethics approval
All animal experiments were approved by the Institutional Review Board of Zhongshan Hospital, Fudan University, and were conducted in accordance with ethical principles governing animal welfare, rearing, and experimentation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Z., Shi, Y., Yin, H. et al. Preliminary study of a novel FAP-targeted ligand 68Ga-DOTA-FL in colon cancer imaging using small-animal PET/CT. Clin Transl Imaging 12, 91–97 (2024). https://doi.org/10.1007/s40336-023-00603-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-023-00603-2