Abstract
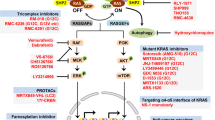
Kirsten rat sarcoma (KRAS) is one of the most frequently mutated oncogenes in solid tumours. It encodes an important signalling pathway that drives cellular proliferation and growth. It is frequently mutated in aggressive advanced solid tumours, particularly colorectal, lung and pancreatic cancer. Since the first mutated KRAS was discovered in the 1980s, decades of research to develop targeted inhibitors of mutant KRAS have fallen short of the task, until recently. Multiple agents are now in clinical trials, including specific mutant KRAS inhibitors, pan-KRAS inhibitors, therapeutic vaccines and other targeted inhibitors. Mutant-specific KRAS G12C inhibitors are the most advanced, with two inhibitors, adagrasib and sotorasib, achieving approval in 2021 for the second-line treatment of patients with KRAS G12C mutant lung cancer. In this review, we summarise the importance of mutant KRAS in solid tumours, prior attempts at inhibiting mutant KRAS, and the current promising targeted agents being investigated in clinical trials, along with future challenges.
Similar content being viewed by others
References
Murugan AK, Grieco M, Tsuchida N. RAS mutations in human cancers: roles in precision medicine. Semin Cancer Biol. 2019;59:23–35.
Barbacid M. RAS genes. Annu Rev Biochem. 1987;56:779–827.
Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17–33.
Tran TH, Chan AH, Young LC, et al. KRAS interaction with RAF1 RAS-binding domain and cysteine-rich domain provides insights into RAS-mediated RAF activation. Nat Commun. 2021;12(1):1176.
Hofmann MH, Gmachl M, Ramharter J, et al. BI-3406, a potent and selective SOS1-KRAS interaction inhibitor, is effective in KRAS-driven cancers through combined MEK inhibition. Cancer Discov. 2021;11(1):142–57.
Downward J. RAS’s cloak of invincibility slips at last? Cancer Cell. 2014;25(1):5–6.
Fernandez-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2(3):344–58.
Santos E, Martin-Zanca D, Reddy EP, Pierotti MA, Della Porta G, Barbacid M. Malignant activation of a K-ras oncogene in lung carcinoma but not in normal tissue of the same patient. Science. 1984;223(4637):661–4.
Forrester K, Almoguera C, Han K, Grizzle WE, Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. Nature. 1987;327(6120):298–303.
Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Biochim Biophys Acta. 2007;1773(8):1177–95.
Rachagani S, Senapati S, Chakraborty S, et al. Activated KrasG(1)(2)D is associated with invasion and metastasis of pancreatic cancer cells through inhibition of E-cadherin. Br J Cancer. 2011;104(6):1038–48.
Li W, Qiu T, Zhi W, et al. Colorectal carcinomas with KRAS codon 12 mutation are associated with more advanced tumor stages. BMC Cancer. 2015;15:340.
Jones RP, Sutton PA, Evans JP, et al. Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer. 2017;116(7):923–9.
Ihle NT, Byers LA, Kim ES, et al. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Natl Cancer Inst. 2012;104(3):228–39.
Valsangkar NP, Ingkakul T, Correa-Gallego C, et al. Survival in ampullary cancer: potential role of different KRAS mutations. Surgery. 2015;157(2):260–8.
Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and susceptibility to cetuximab and panitumumab in colorectal cancer. Cancer J. 2009;15(2):110–3.
Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2011;39(Database issue):D945-950.
Aacr Project GENIE Consortium. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):818–31.
Gautschi O, Huegli B, Ziegler A, et al. Origin and prognostic value of circulating KRAS mutations in lung cancer patients. Cancer Lett. 2007;254(2):265–73.
Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92(1):131–9.
Salgia R, Pharaon R, Mambetsariev I, Nam A, Sattler M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep Med. 2021;2(1):100186.
Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014;13(11):828–51.
Wiesweg M, Kasper S, Worm K, et al. Impact of RAS mutation subtype on clinical outcome-a cross-entity comparison of patients with advanced non-small cell lung cancer and colorectal cancer. Oncogene. 2019;38(16):2953–66.
Nash GM, Gimbel M, Shia J, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17(2):572–8.
Cejas P, Lopez-Gomez M, Aguayo C, et al. KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS ONE. 2009;4(12):e8199.
Conlin A, Smith G, Carey FA, Wolf CR, Steele RJ. The prognostic significance of K-ras, p53, and APC mutations in colorectal carcinoma. Gut. 2005;54(9):1283–6.
Price TJ, Piantadosi C, Townsend AR, et al. Prognostic differences of RAS mutations: Results from South Australian (SA) metastatic colorectal (mCRC) registry. J Clin Oncol. 2020;38(suppl):abstract 4067.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20(10):1218–49.
Hong DS, Fakih MG, Strickler JH, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–17.
Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–51.
Patricelli MP, Janes MR, Li LS, et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 2016;6(3):316–29.
Janes MR, Zhang J, Li LS, et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell. 2018;172(3):578–589517.
Goebel L, Muller MP, Goody RS, Rauh D. KRasG12C inhibitors in clinical trials: a short historical perspective. RSC Med Chem. 2020;11(7):760–70.
Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–23.
Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381.
Skoulidis F, Li BT, Govindan R, et al. Overall survival and exploratory subgroup analyses from the phase 2 CodeBreaK 100 trial evaluating sotorasib in pretreated KRAS p.G12C mutated non-small cell lung cancer. J Clin Oncol. 2021;39:abstract 9003.
Spira AI, Wilson FH, Shapiro GI, et al. Patient-reported outcomes (PRO) from the phase 2 CodeBreaK 100 trial evaluating sotorasib in KRAS p.G12C mutated non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39:abstract 9057.
Fakih MG, Falchook GS, Hong DS, et al. CodeBreaK 101 subprotocol H: Phase Ib study evaluating combination of sotorasib (Soto), a KRASG12C inhibitor, and panitumumab (PMab), an EGFR inhibitor, in advanced KRAS p.G12C-mutated colorectal cancer (CRC). Ann Oncol. 2021;32(suppl_5):S530-582.
Janne PA, Rybkin II, Spira AI, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in advanced/metastatic non-small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur J Cancer. 2020;138(Suppl 2 (abstract)):S2.
Riely GJ, Rybkin II, Spira AI, et al. KRYSTAL-1: activity and preliminary pharmacodynamic (PD) analysis of adagrasib (MRTX849) in patients (Pts) with advanced non-small-cell lung cancer (NSCLC) harboring KRASG12C mutation. J Thorac Oncol. 2021;16:abstract 990_PR.
Weiss J, Yaeger R, Johnson M, et al. KRYSTAL-1: Adagrasib (MRTX849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (CRC) harboring a KRASG12C mutation. Ann Oncol. 2021;32(suppl_5):S1283–346.
Johnson ML, Ou SHI, Barve M, et al. KRYSTAL-1: Activity and Safety of Adagrasib (MRTX849) in Patients with Colorectal Cancer (CRC) and Other Solid Tumors Harboring a KRAS G12C Mutation. Eur J Cancer. 2020;138(Suppl 2 (abstract)):S2.
Sabari JK, Park H, Tolcher AW, et al. KRYSTAL-2: A phase I/II trial of adagrasib (MRTX849) in combination with TNO155 in patients with advanced solid tumors with KRAS G12C mutation. J Clin Oncol. 2021;39(3):TPS146.
NIH U.S National Library of Medicine. A Study to Evaluate the Safety, Pharmacokinetics, and Activity of GDC-6036 alone or in combination in participants with advanced or metastatic solid tumors with a KRAS G12C mutation. (Ed.^(Eds)
Ahronian LG, Corcoran RB. Strategies for monitoring and combating resistance to combination kinase inhibitors for cancer therapy. Genome Med. 2017;9(1):37.
Awad MM, Liu S, Rybkin II, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med. 2021;384(25):2382–93.
Koga T, Suda K, Fujino T, et al. KRAS secondary mutations that confer acquired resistance to KRAS G12C inhibitors, sotorasib and adagrasib, and overcoming strategies: insights from in vitro experiments. J Thorac Oncol. 2021;16(8):1321–32.
Amodio V, Yaeger R, Arcella P, et al. EGFR blockade reverts resistance to KRAS(G12C) inhibition in colorectal cancer. Cancer Discov. 2020;10(8):1129–39.
Fedele C, Li S, Teng KW, et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J Exp Med. 2021. https://doi.org/10.1084/jem.20201414.
Revolution Medicines. RAS(ON) Inhibitors. (Ed.^(Eds) (2021)
Revolution Medicines. Press release: Revolution Medicines Reports Second Quarter Financial Results and Update on Corporate Progress. (Ed.^(Eds) (2021)
Xie M, Xu X, Fan Y. KRAS-mutant non-small cell lung cancer: an emerging promisingly treatable subgroup. Front Oncol. 2021;11:672612.
Johnson ML, Gort E, Pant S, et al. A phase I, open-label, dose-escalation trial of BI 1701963 in patients (pts) with KRAS mutated solid tumours: a snapshot analysis. Ann Oncol. 2021;32(suppl_5):S583-620.
Brana I, Shapiro GI, Johnson ML, et al. Initial results from a dose finding study of TNO155, a SHP2 inhibitor, in adults with advanced solid tumors. J Clin Oncol. 2021;39:abstract 3005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding or financial assistance was provided for the development of this article.
Conflicts of interest
Tharani Krishnan has no conflicts of interest to disclose. Rachel Roberts-Thomson: Speakers fees for MSD, BMS, Astra-Zeneca, Roche and Novartis. Vy Broadbridge has no conflicts of interest to disclose. Timothy Price: AMGEN, advisory board non compensated.
Ethics approval
Not applicable.
Author contributions
All the authors made substantial contributions to the planning, research and writing of this review article.
Data availability statement
Not applicable.
Rights and permissions
About this article
Cite this article
Krishnan, T., Roberts-Thomson, R., Broadbridge, V. et al. Targeting Mutated KRAS Genes to Treat Solid Tumours. Mol Diagn Ther 26, 39–49 (2022). https://doi.org/10.1007/s40291-021-00564-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-021-00564-0