Abstract
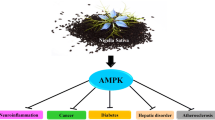
Diabetes mellitus represents a chronic metabolic disorder characterized by impaired lipid homeostasis and carbohydrate metabolism, gradually leading to persistent hyperglycemia. The extracts of Rhodiola species are widely used as herbal medicine or dietary supplement in Asia, Europe and the United States. Salidroside, a p-hydroxyphenethyl-β-glucoside compound, is the main active ingredient of the Rhodiola root. Recently, various studies have suggested that Rhodiola and salidroside may have pharmacological properties that could be used in the treatment of diabetes, as studies have confirmed that AMP-activated protein kinase (AMPK) and AMPK-related signaling are connected with its beneficial effects. This review aims to summarize the research progress of Rhodiola and salidroside in the treatment of diabetes. A detailed summary of AMPK and AMPK-related signaling induced by Rhodiola and salidroside are discussed.


Similar content being viewed by others
References
American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37(1):11–34.
Mendis S, Armstrong T, Bettcher D, Branca F, Lauer J, Mace C, et al. Global status report on noncommunicable diseases 2014. Geneva: World Health Organization; 2015.
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–49.
Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7.
Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6(3):293–302.
Panossian A, Wagner H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005;19(10):819–38.
Booker A, Zhai L, Gkouva C, Li S, Heinrich M. From traditional resource to global commodities: a comparison of Rhodiola species using NMR spectroscopy-metabolomics and HPTLC. Front Pharmacol. 2016;7:254.
Chiang HM, Chen HC, Wu CS, Wu PY, Wen KC. Rhodiola plants: chemistry and biological activity. J Food Drug Anal. 2015;23(3):359–69.
Xin T, Li X, Yao H, Lin Y, Ma X, Cheng R, et al. Survey of commercial Rhodiola products revealed species diversity and potential safety issues. Sci Rep. 2015;9(5):8337.
Zhang ZH, Feng SH, Hu GD, Cao ZK, Wang LY. Effect of Rhodiola kirilowii (Regel.) Maxim on preventing high altitude reactions. A comparison of cardiopulmonary function in villagers at various altitudes. China J Chin Mater Med. 1989;14(11):687–90, 704.
Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17(7):481–93.
Ali Z, Fronczek FR, Khan IA. Phenylalkanoids and monoterpene analogues from the roots of Rhodiola rosea. Planta Med. 2008;74(2):178–81.
Grech-Baran M, Syklowska-Baranek K, Pietrosiuk A. Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp. in vitro cultures. Phytochem Rev. 2015;14(4):657–74.
Qian EW, Ge DT, Kong S-K. Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin. J Ethnopharmacol. 2011;133(2):308–14.
Mao GX, Deng HB, Yuan LG, Li DD, Li YY, Wang Z. Protective role of salidroside against aging in a mouse model induced by d-galactose. Biomed Environ Sci. 2010;23(2):161–6.
Wang J, Li JZ, Lu AX, Zhang KF, Li BJ. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol Lett. 2014;7(4):1159–64.
Zhang J, Zhen Y-F, Song L-G, Kong W-N, Shao T-M, Li X, et al. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav Brain Res. 2013;244:70–81.
Chen J-J, Zhang N-F, Mao G-X, He X-B, Zhan Y-C, Deng H-B, et al. Salidroside stimulates osteoblast differentiation through BMP signaling pathway. Food Chem Toxicol. 2013;62:499–505.
Zheng T, Yang X, Wu D, Xing S, Bian F, Li W, et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3beta pathway. Br J Pharmacol. 2015;172(13):3284–301.
Wang S, Zhao X, Yang S, Chen B, Shi J. Salidroside alleviates high glucose-induced oxidative stress and extracellular matrix accumulation in rat glomerular mesangial cells by the TXNIP-NLRP3 inflammasome pathway. Chem Biol Interact. 2017;25(278):48–53.
Alameddine A, Fajloun Z, Bourreau J, Gauquelin-Koch G, Yuan M, Gauguier D, et al. The cardiovascular effects of salidroside in the Goto-Kakizaki diabetic rat model. J Physiol Pharmacol. 2015;66(2):249–57.
Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7.
Wu D, Yang X, Zheng T, Xing S, Wang J, Chi J, et al. A novel mechanism of action for salidroside to alleviate diabetic albuminuria: effects on albumin transcytosis across glomerular endothelial cells. Am J Physiol Endocrinol Metab. 2016;310(3):E225–37.
Zheng T, Yang X, Li W, Wang Q, Chen L, Wu D, et al. Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid Med Cell Longev. 2018;2018:8597897.
Zhang YL, Guo H, Zhang CS, Lin SY, Yin Z, Peng Y, et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN–AMPK–LKB1 complex for AMPK activation. Cell Metab. 2013;18(4):546–55.
Suzuki T, Bridges D, Nakada D, Skiniotis G, Morrison SJ, Lin JD, et al. Inhibition of AMPK catabolic action by GSK3. Mol Cell. 2013;50(3):407–19.
Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62.
Witters LA, Kemp BE, Means AR. Chutes and ladders: the search for protein kinases that act on AMPK. Trends Biochem Sci. 2006;31(1):13–6.
Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–23.
Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, et al. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332(6036):1433–5.
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–35.
Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–81.
Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65(9):2784–94.
Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66(6):789–800.
Cheng X, Di L, Wu Y, Zhao Q, Du G, Liu Y. Studies on the hypoglycemic effect of Rhodiola sachalinensis A. Bor. polysaccharides. China J Chin Mater Med. 1993;18(9):557–9.
Gao D, Li Q, Liu Z, Feng J, Li J, Han Z, et al. Antidiabetic potential of Rhodiola sachalinensis root extract in streptozotocin-induced diabetic rats. Methods Find Exp Clin Pharmacol. 2009;31(6):375–81.
Mao Y. Hypoglycemic and hypolipidaemic activities of polysaccharides from Rhodiola rosea in KKAy mice. J Food Process Preserv. 2017;41(6):e13219.
Kwon YI, Jang HD, Shetty K. Evaluation of Rhodiola crenulata and Rhodiola rosea for management of type II diabetes and hypertension. Asia Pac J Clin Nutr. 2006;15(3):425–32.
Kwon Y-I, Apostolidis E, Shetty K. Anti-diabetes functionality of Kefir culture-mediated fermented soymilk supplemented with Rhodiola extracts. Food Biotechnol. 2006;20(1):13–29.
Kim SH, Hyun SH, Choung SY. Antioxidative effects of Cinnamomi cassiae and Rhodiola rosea extracts in liver of diabetic mice. Biofactors. 2006;26(3):209–19.
Park C, Lee J-S. Mini review: natural ingredients for diabetes which are approved by Korean FDA. Biomed Res. 2013;24(1):164–9.
Wang J, Rong X, Li W, Yang Y, Yamahara J, Li Y. Rhodiola crenulata root ameliorates derangements of glucose and lipid metabolism in a rat model of the metabolic syndrome and type 2 diabetes. J Ethnopharmacol. 2012;142(3):782–8.
Lee SY, Lai FY, Shi LS, Chou YC, Yen IC, Chang TC. Rhodiola crenulata extract suppresses hepatic gluconeogenesis via activation of the AMPK pathway. Phytomedicine. 2015;22(4):477–86.
Cheng YZ, Chen LJ, Lee WJ, Chen MF, Jung Lin H, Cheng JT. Increase of myocardial performance by Rhodiola–ethanol extract in diabetic rats. J Ethnopharmacol. 2012;144(2):234–9.
Gallagher H, Suckling RJ. Diabetic nephropathy: where are we on the journey from pathophysiology to treatment? Diabetes Obes Metab. 2016;18(7):641–7.
Wang Z, Gao F, Lu F-E. Effect of ethanol extract of Rhodiola rosea on the early nephropathy in type 2 diabetic rats. J Huazhong Univ Sci Technol [Med Sci]. 2013;33:375–8.
Li HB, Ge YK, Zheng XX, Zhang L. Salidroside stimulated glucose uptake in skeletal muscle cells by activating AMP-activated protein kinase. Eur J Pharmacol. 2008;588(2–3):165–9.
Li F, Tang H, Xiao F, Gong J, Peng Y, Meng X. Protective effect of salidroside from Rhodiolae radix on diabetes-induced oxidative stress in mice. Molecules. 2011;16(12):9912–24.
Wang M, Luo L, Yao L, Wang C, Jiang K, Liu X, et al. Salidroside improves glucose homeostasis in obese mice by repressing inflammation in white adipose tissues and improving leptin sensitivity in hypothalamus. Sci Rep. 2016;5(6):25399.
Zhang XR, Fu XJ, Zhu DS, Zhang CZ, Hou S, Li M, et al. Salidroside-regulated lipid metabolism with down-regulation of miR-370 in type 2 diabetic mice. Eur J Pharmacol. 2016;15(779):46–52.
Ma YG, Wang JW, Bai YG, Liu M, Xie MJ, Dai ZJ. Salidroside contributes to reducing blood pressure and alleviating cerebrovascular contractile activity in diabetic Goto-Kakizaki rats by inhibition of L-type calcium channel in smooth muscle cells. BMC Pharmacol Toxicol. 2017;18(1):30.
Ma YG, Wang JW, Zhang YB, Wang BF, Dai ZJ, Xie MJ, et al. Salidroside improved cerebrovascular vasodilation in streptozotocin-induced diabetic rats through restoring the function of BKCa channel in smooth muscle cells. Cell Tissue Res. 2017;370(3):365–77.
Zhang P, Li Y, Guo R, Zang W. Salidroside protects against advanced glycation end products-induced vascular endothelial dysfunction. Med Sci Monit. 2018;21(24):2420–8.
Xing SS, Yang XY, Zheng T, Li WJ, Wu D, Chi JY, et al. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vascul Pharmacol. 2015;72:141–52.
Ni GL, Cui R, Shao AM, Wu ZM. Salidroside ameliorates diabetic neuropathic pain in rats by inhibiting neuroinflammation. J Mol Neurosci. 2017;63(1):9–16.
Zhao H, Zhang Y, Liu B, Meng K, Wang C. Effects of salidroside on the protection of diabetic encephalopathy and improvement of abilities of learning and memory in rat. J Med Plants Res. 2011;5(27):6328–35.
Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806.
Wang SH, Wang WJ, Wang XF, Chen WH. Effects of salidroside on carbohydrate metabolism and differentiation of 3T3-L1 adipocytes. J Chin Integr Med. 2004;2(3):193–5.
Lin KT, Hsu SW, Lai FY, Chang TC, Shi LS, Lee SY. Rhodiola crenulata extract regulates hepatic glycogen and lipid metabolism via activation of the AMPK pathway. BMC Complement Altern Med. 2016;17(16):127.
Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9(5):407–16.
Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–5.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Investig. 2004;114(12):1752–61.
Bian F, Yang X, Zhou F, Wu PH, Xing S, Xu G, et al. C-reactive protein promotes atherosclerosis by increasing LDL transcytosis across endothelial cells. Br J Pharmacol. 2014;171(10):2671–84.
Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu G, et al. TNF-alpha promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-kappaB and PPAR-gamma. J Mol Cell Cardiol. 2014;72:85–94.
Xing S, Yang X, Li W, Bian F, Wu D, Chi J, et al. Salidroside stimulates mitochondrial biogenesis and protects against H(2)O(2)-induced endothelial dysfunction. Oxid Med Cell Longev. 2014;2014:904834.
Yin D, Yao W, Chen S, Hu R, Gao X. Salidroside, the main active compound of Rhodiola plants, inhibits high glucose-induced mesangial cell proliferation. Planta Med. 2009;75(11):1191–5.
Lu H, Li Y, Zhang T, Liu M, Chi Y, Liu S, et al. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upregulating heme oxygenase-1 (HO-1) expression. Med Sci Monit. 2017;23(23):4067–76.
Liu XM, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol. 2011;300(1):H84–93.
Marasco MR, Conteh AM, Reissaus CA, Cupit JET, Appleman EM, Mirmira RG, et al. Interleukin-6 reduces beta-cell oxidative stress by linking autophagy with the antioxidant response. Diabetes. 2018;67(8):1576–88.
Ju L, Wen X, Wang C, Wei Y, Peng Y, Ding Y, et al. Salidroside, a natural antioxidant, improves beta-cell survival and function via activating AMPK pathway. Front Pharmacol. 2017;8:749.
Huang LY, Yen IC, Tsai WC, Ahmetaj-Shala B, Chang TC, Tsai CS, et al. Rhodiola crenulata attenuates high glucose induced endothelial dysfunction in human umbilical vein endothelial cells. Am J Chin Med. 2017;45(6):1201–16.
Zheng XT, Wu ZH, Wei Y, Dai JJ, Yu GF, Yuan F, et al. Induction of autophagy by salidroside through the AMPK-mTOR pathway protects vascular endothelial cells from oxidative stress-induced apoptosis. Mol Cell Biochem. 2017;425(1–2):125–38.
Ruiz R, Perez-Villegas EM, Manuel Carrion A. AMPK function in aging process. Curr Drug Targets. 2016;17(8):932–41.
Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–64.
Dun Y, Liu S, Zhang W, Xie M, Qiu L. Exercise combined with Rhodiola sacra supplementation improves exercise capacity and ameliorates exhaustive exercise-induced muscle damage through enhancement of mitochondrial quality control. Oxid Med Cell Longev. 2017;2017:8024857.
Gospodaryov DV, Yurkevych IS, Jafari M, Lushchak VI, Lushchak OV. Lifespan extension and delay of age-related functional decline caused by Rhodiola rosea depends on dietary macronutrient balance. Longev Healthspan. 2013;2(1):5.
Xing SS, Li J, Chen L, Yang YF, He PL, Yang J. Salidroside attenuates endothelial cellular senescence via decreasing the expression of inflammatory cytokines and increasing the expression of SIRT3. Mech Ageing Dev. 2017;28(175):1–6.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–60.
Xue H, Li P, Luo Y, Wu C, Liu Y, Qin X, et al. Salidroside stimulates the Sirt1/PGC-1alpha axis and ameliorates diabetic nephropathy in mice. Phytomedicine. 2019;15(54):240–7.
Zhang Y, Ye M, Wang X, Wang G, Yang H, Ma J. Effect of salidroside on PI3K/protein kinase B signaling in Parkinson’s disease model mice. J Clin Neurol. 2008;21:133–5.
Chen SF, Tsai HJ, Hung TH, Chen CC, Lee CY, Wu CH, et al. Salidroside improves behavioral and histological outcomes and reduces apoptosis via PI3K/Akt signaling after experimental traumatic brain injury. PLoS One. 2012;7(9):e45763.
Xu M-C, Shi H-M, Gao X-F, Wang H. Salidroside attenuates myocardial ischemia–reperfusion injury via PI3K/Akt signaling pathway. J Asian Nat Prod Res. 2013;15(3):244–52.
Booker A, Jalil B, Frommenwiler D, Reich E, Zhai L, Kulic Z, et al. The authenticity and quality of Rhodiola rosea products. Phytomedicine. 2016;23(7):754–62.
Martel J, Ko YF, Ojcius DM, Lu CC, Chang CJ, Lin CS, et al. Immunomodulatory properties of plants and mushrooms. Trends Pharmacol Sci. 2017;38(11):967–81.
Chandramohan R, Pari L, Rathinam A, Sheikh BA. Tyrosol, a phenolic compound, ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Chem Biol Interact. 2015;5(229):44–54.
Punithavathi VR, Prince PS, Kumar R, Selvakumari J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol. 2011;650(1):465–71.
Zhang Y, Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur J Pharmacol. 2011;670(1):325–32.
Jiao L, Zhang X, Huang L, Gong H, Cheng B, Sun Y, et al. Proanthocyanidins are the major anti-diabetic components of cinnamon water extract. Food Chem Toxicol. 2013;56:398–405.
Veeramani C, Alsaif MA, Al-Numair KS. Herbacetin, a flaxseed flavonoid, ameliorates high percent dietary fat induced insulin resistance and lipid accumulation through the regulation of hepatic lipid metabolizing and lipid-regulating enzymes. Chem Biol Interact. 2018;25(288):49–56.
Author information
Authors and Affiliations
Contributions
SJ and TZ conceived and designed the study. TZ, FB, LC, QW and SJ reviewed the data, drew the figure and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
TZ, FB, LC, QW and SJ have no conflicts of interest that are directly relevant to the content of this article.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81703582, 81573432, 81503072 and 81373413), Ministry of Education of China (NCET-10-0409), and Hubei Provincial Natural Science Foundation of China (2016CFB153).
Rights and permissions
About this article
Cite this article
Zheng, T., Bian, F., Chen, L. et al. Beneficial Effects of Rhodiola and Salidroside in Diabetes: Potential Role of AMP-Activated Protein Kinase. Mol Diagn Ther 23, 489–498 (2019). https://doi.org/10.1007/s40291-019-00402-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-019-00402-4