Abstract
Background
Testosterone, cortisol and their ratios may be indicators of anabolic status, but technical issues surrounding blood sampling has limited wider application. The advent of salivary testosterone (sal-T) analysis simplified sample acquisition, resulting in a subsequent rapid increase in the number of published research articles.
Objective
The objective of this study was to undertake a meta-analysis to determine the effect of acute exercise bouts on post exercise sal-T and salivary cortisol (sal-C) concentrations and their ratio (sal-T:C).
Data Sources
Relevant databases such as PubMed, Web of Science, Science Direct and SPORTDiscus were searched up to and including 31 December 2013 for the term ‘saliva AND testosterone AND exercise’.
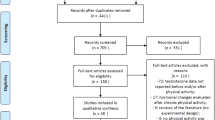
Study Selection
Studies (n = 21) selected from the 933 identified included randomised controlled trials (RCTs; n = 2), uncontrolled trials (UCTs; n = 18) and control trials (CTs; n = 1), all of which had an exercise component characterised as either aerobic, resistance or power training, each with acute sal-T and sal-C measurement obtained within 30 min of exercise bout completion.
Study Appraisal and Synthesis Methods
A meta-analysis was conducted on change in sal-T, sal-C and the sal-T:C ratio following exercise using standard difference in means (SDM) and a random effects model.
Results
For aerobic, resistance and power exercise, the overall SDMs for sal-T were 0.891, 1.061 and 0.509, respectively; for sal-C, the SDMs were 3.041, 0.773 and 1.200, respectively. For sal-T:C, the SDMs were −2.014, 0.027 and −0.968, respectively. RCTs, UCTs and CTs were separated by subgroup analysis. There were significant differences in overall weighted SDM values for sal-T between RCTs, UCTs and CTs within exercise modes. When examining aerobic exercise interventions, a quantitative interaction of study design was observed. RCTs resulted in a greater SDM than UCTs (1.337 vs. 0.446). Power interventions displayed a qualitative interaction with study design. UCTs where baseline measures were obtained 24 h before exercise had an SDM of –1.128, whereas UCTs where baseline was determined immediately prior to exercise had an SDM of 0.486. The single CT trial had an SDM of 2.260. Resistance exercise interventions were primarily UCTs; however, an observed influence of baseline sampling time whereby immediately pre- and 24 h pre-exercise resulted in differing SDMs. The sole resistance exercise RCTs resulted in the greatest SDM (2.500).
Conclusion
The current body of evidence regarding acute responses of sal-T to exercise is weak. This meta-analysis identifies varying exercise-dependent effect sizes. Each appear to be greatly influenced by study design and sample timing. There is a need for more RCTs and a standardised methodology for the measurement of salivary hormones in order to better determine the effect of exercise modality.




Similar content being viewed by others
References
Hayes LD, Grace FM, Sculthorpe N, et al. Does chronic exercise attenuate age-related physiological decline in males? Res Sport Med. 2013;21(4):343–54.
Groschl M. The physiological role of hormones in saliva. Bioessays. 2009;31(8):843–52.
Caruso JF, Lutz BM, Davidson ME, et al. Salivary hormonal values from high-speed resistive exercise workouts. J Strength Cond Res. 2012;26(3):625–32.
Chang CK, Tseng HF, Tan HF, et al. Responses of saliva testosterone, cortisol, and testosterone-to-cortisol ratio to a triathlon in young and middle-aged males. Biol Sport. 2005;22(3):227–35.
Lewis JG. Steroid analysis in saliva: an overview. Clin Biochem Rev. 2006;27(3):139–46.
Cook CJ, Kilduff LP, Beaven CM. Improving strength and power in trained athletes with 3 weeks of occlusion training. Int J Sport Physiol Perform. 2014;9(1):166–72.
Crewther BT, Sanctuary CE, Kilduff LP, et al. The workout responses of salivary-free testosterone and cortisol concentrations and their association with the subsequent competition outcomes in professional rugby league. J Strength Cond Res. 2013;27(2):471–6.
Duclos M. A critical assessment of hormonal methods used in monitoring training status in athletes. Int Sportmed J. 2008;9(2):56–66.
Slivka DR, Hailes WS, Cuddy JS, et al. Effects of 21 days of intensified training on markers of overtraining. J Strength Cond Res. 2010;24(10):2604–12.
Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome-induced proteolysis in skeletal muscle by beta-hydroxy-beta-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005;65(1):277–83.
Lac G, Maso F. Biological markers for the follow-up of athletes throughout the training season. Pathol Biol. 2004;52(1):43–9.
Maso F, Lac G, Filaire E, et al. Salivary testosterone and cortisol in rugby players: correlation with psychological overtraining items. Br J Sport Med. 2004;38(3):260–3.
Beaven CM, Cook CJ, Gill ND. Significant strength gains observed in rugby players after specific resistance exercise protocols based on individual salivary testosterone responses. J Strength Cond Res. 2008;22(2):419–25.
Beaven CM, Gill ND, Cook CJ. Salivary testosterone and cortisol responses in professional rugby players after four resistance exercise protocols. J Strength Cond Res. 2008;22(2):426–32.
Beaven CM, Gill ND, Ingram JR, et al. Acute salivary hormone responses to complex exercise bouts. J Strength Cond Res. 2011;25(4):1072–8.
Cook CJ, Crewther BT. Changes in salivary testosterone concentrations and subsequent voluntary squat performance following the presentation of short video clips. Horm Behav. 2012;161(1):17–22.
Cook CJ, Kilduff LP, Crewther BT, et al. Morning based strength training improves afternoon physical performance in rugby union players. J Sci Med Sport. 2013;17(3):317–21.
Kilduff L, Cook CJ, Bennett M, et al. Right-left digit ratio (2D:4D) predicts free testosterone levels associated with a physical challenge. J Sport Sci. 2013;31(6):677–83.
Cook CJ, Crewther BT, Kilduff LP. Are free testosterone and cortisol concentrations associated with training motivation in elite male athletes? Psychol Sport Exerc. 2013;14(6):882–5.
Cook CJ, Crewther BT. The effects of different pre-game motivational interventions on athlete free hormonal state and subsequent performance in professional rugby union matches. Physiol Behav. 2012;106(5):683–8.
Cook CJ, Crewther BT, Smith AA. Comparison of baseline free testosterone and cortisol concentrations between elite and non-elite female athletes. Am J Human Biol. 2012;24(6):856–8.
Crewther B, Cronin J, Keogh J, et al. The salivary testosterone and cortisol response to three loading schemes. J Strength Cond Res. 2008;22(1):250–5.
Crewther BT, Kilduff LP, Cook CJ, et al. The acute potentiating effects of back squats on athlete performance. J Strength Cond Res. 2011;25(12):3319–25.
Crewther BT, Kilduff LP, Cook CJ, et al. Relationships between salivary free testosterone and the expression of force and power in elite athletes. J Sport Med Phys Fit. 2012;52(2):221–7.
Crewther BT, Cook CJ, Lowe TE, et al. The effects of short-cycle sprints on power, strength, and salivary hormones in elite rugby players. J Strength Cond Res. 2011;25(1):32–9.
Paton CD, Hopkins WG, Cook C. Effects of high- vs low-cadence interval training on physiology and performance of competitive cyclists [poster]. Med Sci Sport Exerc. 2006;38(Suppl. 5):S490.
Hayes LD, Grace FM, Sculthorpe N, et al. The effects of a formal exercise training programme on salivary hormone concentrations and body composition in previously sedentary aging men. SpringerPlus. 2013;2(1):18.
Baillot A, Vibarel-Rebot N, Amiot V, et al. Effects of an 8-week aerobic exercise training on saliva steroid hormones, physical capacity, and quality of life in diabetic obese men. Horm Metab Res. 2012;44(2):146–51.
Elloumi M, Maso F, Michaux O, et al. Behaviour of saliva cortisol C, testosterone T and the T/C ratio during a rugby match and during the post-competition recovery days. Eur J Appl Physiol. 2003;90(1–2):23–8.
Elloumi M, Maso F, Robert A, et al. Saliva cortisol and testosterone values during the week following a match in rugbymen. Sci Sport. 2003;18(3):164–5.
Gomes RV, Moreira A, Lodo L, et al. Monitoring training loads, stress, immune-endocrine responses and performance in tennis players. Biol Sport. 2013;30(3):173–80.
West DJ, Cunningham DJ, Finn C, et al. The metabolic, hormonal, biochemical and neuromuscular function responses to a backward sled drag training session. J Strength Cond Res. 2014;28(1):265–72.
West DJ, Finn C, Cunningham DJ, et al. The neuromuscular function, hormonal, and mood responses to a professional rugby union match. J Strength Cond Res. 2014;28(1):194–200.
McLellan CP, Lovell DI, Gass GC. Creatine kinase and endocrine responses of elite players pre, during, and post rugby league match play. J Strength Cond Res. 2010;24(11):2908–19.
Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–61.
Crewther B, Keogh J, Cronin J, et al. Possible stimuli for strength and power adaptation: acute hormonal responses. Sports Med. 2006;36(3):215–38.
Mitchell CJ, Churchward-Venne TA, Bellamy L, et al. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PloS One. 2013;8(10):e78636.
Schroeder ET, Villanueva M, West DD, et al. Are acute post-resistance exercise increases in testosterone, growth hormone, and IGF-1 necessary to stimulate skeletal muscle anabolism and hypertrophy? Med Sci Sports Exerc. 2013;45(11):2044–51.
West DWD, Burd NA, Tang JE, et al. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol. 2010;108(1):60–7.
Wilkinson SB, Tarnopolsky MA, Grant EJ, et al. Hypertrophy with unilateral resistance exercise occurs without increases in endogenous anabolic hormone concentration. Eur J Appl Physiol. 2006;98(6):546–55.
Gonzalez-Bono E, Moya-Albiol L, Martinez-Sanchis S, et al. Salivary testosterone and cortisol responses to cycle ergometry in basketball players with different training volume. J Psychophysiol. 2002;16(3):158–66.
Granger DA, Shirtcliff EA, Booth A, et al. The “trouble” with salivary testosterone. Psychoneuroendocrinology. 2004;29(10):1229–40.
Wahl P, Mathes S, Kohler K, et al. Acute metabolic, hormonal, and psychological responses to different endurance training protocols. Horm Metab Res. 2013;45(11):827–33.
Landman AD, Sanford LM, Howland BE, et al. Testosterone in human saliva. Experientia. 1976;32(7):940–1.
de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–33.
Hough JP, Papacosta E, Wraith E, et al. Plasma and salivary steroid hormone responses of men to high-intensity cycling and resistance exercise. J Strength Cond Res. 2011;25(1):23–31.
Hough J, Corney R, Kouris A, et al. Salivary cortisol and testosterone responses to high-intensity cycling before and after an 11-day intensified training period. J Sport Sci. 2013;31(14):1614–23.
Doan BK, Newton RU, Kraemer WJ, et al. Salivary cortisol, testosterone, and T/C ratio responses during a 36-hole golf competition. Int J Sport Med. 2007;28(6):470–9.
Crewther BT, Lowe TE, Ingram J, et al. Validating the salivary testosterone and cortisol concentration measures in response to short high-intensity exercise. J Sport Med Phys Fit. 2010;50(1):85–92.
Thorpe R, Sunderland C. Muscle damage, endocrine, and immune marker response to a soccer match. J Strength Cond Res. 2012;26(10):2783–90.
Cadore E, Lhullier F, Brentano M, et al. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J Sport Sci. 2008;26(10):1067–72.
Ghigiarelli JJ, Sell KM, Raddock JM, et al. Effects of strongman training on salivary testosterone levels in a sample of trained men. J Strength Cond Res. 2013;27(3):738–47.
Crewther BT, Lowe T, Weatherby RP, et al. Prior sprint cycling did not enhance training adaptation, but resting salivary hormones were related to workout power and strength. Eur J Appl Physiol. 2009;105(6):919–27.
Le Panse B, Vibarel-Rebot N, Parage G, et al. Cortisol, DHEA, and testosterone concentrations in saliva in response to an international powerlifting competition. Stress. 2010;13(6):528–32.
Le Panse B, Labsy Z, Baillot A, et al. Changes in steroid hormones during an international powerlifting competition. Steroids. 2012;77(13):1339–44.
McLellan CP, Lovell DI, Gass GC. Biochemical and endocrine responses to impact and collision during elite Rugby League match play. J Strength Cond Res. 2011;25(6):1553–62.
Kraemer WJ. Exercise prescription in weight training: manipulating program variables. Strength Cond J. 1983;5(3):58–61.
Hakkinen K, Pakarinen A. Acute hormonal responses to 2 different fatiguing heavy-resistance protocols in male-athletes. J Appl Physiol. 1993;74(2):882–7.
Hakkinen K, Pakarinen A. Serum hormones in male strength athletes during intensive short-term strength training. Eur J Appl Physiol Occup Physiol. 1991;63(3–4):194–9.
Hakkinen K, Pakarinen A, Kraemer WJ, et al. Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J Appl Physiol. 2001;91(2):569–80.
Fleming AS, Corter C, Stallings J, et al. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42(4):399–413.
Cook CJ, Crewther BT. Changes in salivary testosterone concentrations and subsequent voluntary squat performance following the presentation of short video clips. Horm Behav. 2012;61(1):17–22.
Valero-Politi J, Fuentes-Arderiu X. Within- and between-subject biological variations of follitropin, lutropin, testosterone, and sex-hormone-binding globulin in men. Clin Chem. 1993;39(8):1723–5.
Hayes LD, Sculthorpe N, Young JD, et al. Critical difference applied to exercise-induced salivary testosterone and cortisol using enzyme-linked immunosorbent assay: distinguishing biological from statistical change. J Physiol Biochem. 2014;70(4):991–6.
Crewther BT, Cook CJ, Gaviglio CM, et al. Baseline strength can influence the ability of salivary free testosterone to predict squat and sprinting performance. J Strength Cond Res. 2012;26(1):261–8.
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no potential conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hayes, L.D., Grace, F.M., Baker, J.S. et al. Exercise-Induced Responses in Salivary Testosterone, Cortisol, and Their Ratios in Men: A Meta-Analysis. Sports Med 45, 713–726 (2015). https://doi.org/10.1007/s40279-015-0306-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-015-0306-y