Abstract
Objective
We aimed to perform an early cost-effectiveness analysis of using a whole-genome sequencing-based tumor mutation burden (WGS-TMB), instead of programmed death-ligand 1 (PD-L1), for immunotherapy treatment selection in patients with non-squamous advanced/metastatic non-small cell lung cancer ineligible for targeted therapy, from a Dutch healthcare perspective.
Methods
A decision-model simulating individual patients with metastatic non-small cell lung cancer was used to evaluate diagnostic strategies to select first-line immunotherapy only or the immunotherapy plus chemotherapy combination. Treatment was selected using PD-L1 [A, current practice], WGS-TMB [B], and both PD-L1 and WGS-TMB [C]. Strategies D, E, and F take into account a patient’s disease burden, in addition to PD-L1, WGS-TMB, and both PD-L1 and WGS-TMB, respectively. Disease burden was defined as a fast-growing tumor, a high number of metastases, and/or weight loss. A threshold of 10 mutations per mega-base was used to classify patients into TMB-high and TMB-low groups. Outcomes were discounted quality-adjusted life-years (QALYs) and healthcare costs measured from the start of first-line treatment to death. Healthcare costs includes drug acquisition, follow-up costs, and molecular diagnostic tests (i.e., standard diagnostic techniques and/or WGS for strategies involving TMB). Results were reported using the net monetary benefit at a willingness-to-pay threshold of €80,000/QALY. Additional scenario and threshold analyses were performed.
Results
Strategy B had the lowest QALYs (1.84) and lowest healthcare costs (€120,800). The highest QALYs and healthcare costs were 2.00 and €140,400 in strategy F. In the base-case analysis, strategy A was cost effective with the highest net monetary benefit (€27,300), followed by strategy B (€26,700). Strategy B was cost effective when the cost of WGS testing was decreased by at least 24% or when immunotherapy results in an additional 0.5 year of life gained or more for TMB high compared with TMB low. Strategies C and F, which combined TMB and PD-L1 had the highest net monetary benefit (≥ €76,900) when the cost of WGS testing, immunotherapy, and chemotherapy acquisition were simultaneously reduced by at least 47%, 39%, and 43%, respectively. Furthermore, strategy C resulted in the highest net monetary benefit (≥ €39,900) in a scenario where patients with both PD-L1 low and TMB low were treated with chemotherapy instead of immunotherapy plus chemotherapy.
Conclusions
The use of WGS-TMB is not cost effective compared to PD-L1 for immunotherapy treatment selection in non-squamous metastatic non-small cell lung cancer in the Netherlands. WGS-TMB could become cost effective provided there is a reduction in the cost of WGS testing or there is an increase in the predictive value of WGS-TMB for immunotherapy effectiveness. Alternatively, a combination strategy of PD-L1 testing with WGS-TMB would be cost effective if used to support the choice to withhold immunotherapy in patients with a low expected benefit of immunotherapy.
Similar content being viewed by others
The costs of whole-genome sequencing testing, immunotherapy, and chemotherapy acquisition are key drivers for the cost-effectiveness of using a whole-genome sequencing tumor mutational burden compared to programmed death ligand 1 for immunotherapy treatment decisions in patients with advanced or metastatic non-small cell lung cancer in the Netherlands. |
Substantial cost savings with a minimal loss in mean quality-adjusted life-years per patient can be achieved if the whole-genome sequencing tumor mutational burden in combination with programmed death ligand 1 is used to support the decision to withhold immunotherapy in patients with the least expected benefit of immunotherapy, i.e., those with both a tumor mutational burden less than 10 mutations per mega-base and expression of programmed death ligand 1 of less than 1%. |
The potential of withholding immunotherapy for patients with the least expected benefit merits further investigation. |
1 Introduction
The treatment scheme for patients with advanced (inoperable/metastatic) non-small cell lung cancer ineligible for local treatment (metNSCLC) has become more and more personalized, with treatment selection depending on therapy-predictive biomarkers [1,2,3,4]. These predictive biomarkers are most common in non-squamous metNSCLC [2, 5]. The selection of appropriate therapy is important because for some patient groups, the 5-year survival rate has been shown to improve from 6% when treated with platinum-based chemotherapy (ChT) to between 15 and 50% when treated with the appropriate targeted therapy or immunotherapy [6].
Therapy-predictive biomarkers divide patients with metNSCLC into two main groups, a group eligible for targeted therapy (i.e., patients with a molecular alteration or aberration that can be therapeutically targeted with approved drugs [2]) and a group ineligible for targeted therapy [1,2,3,4]. The group ineligible for targeted therapy forms the largest subgroup, about 75% of patients with metNSCLC in the Netherlands [5]. Immunotherapy such as pembrolizumab (with or without ChT) is the recommended first-line treatment for patients ineligible for targeted therapy [1,2,3,4]. Nevertheless, the response rate to immunotherapy remains low, about 20% [7, 8]. Immunotherapy is also associated with a risk of immune-related adverse events, and to some patients, a worsening of disease outcome [9, 10]. Furthermore, immunotherapy is expensive costing up to 100,000 Euros per patient [11].
The response to immunotherapy is heterogeneous. A better response to immunotherapy has been observed for higher levels of programmed death-ligand 1 (PD-L1) expression [12, 13]. Consequently, PD-L1 expression received approval as a predictive biomarker in the USA and in Europe [14,15,16]. While patients with PD-L1 of at least 50% expression receive immunotherapy monotherapy, those with PD-L1 less than 50% receive additional ChT with the immunotherapy [1,2,3,4]. Although PD-L1 expression is a predictor for survival at the population level, at the individual patient level, it is an imperfect biomarker to classify responders versus non responders [9].
There is a need for a better predictive biomarker for immunotherapy response than PD-L1 expression. One of the most studied potential biomarkers is tumor mutational burden (TMB). Tumor mutational burden is measured as the number of somatic mutations per genome mega-base pair (mut/Mb) using next-generation sequencing (NGS) techniques, i.e., NGS panels, whole-exome sequencing, or whole-genome sequencing (WGS) [17]. Whole-genome sequencing-based TMB is the most reliable measurement and considered as the golden standard [18, 19]. The potential predictive role of TMB was shown for different immunotherapies such as pembrolizumab, nivolumab, and nivolumab plus ipilimumab [20,21,22,23]. Furthermore, it has been shown that the TMB level and PD-L1 expression are independent predictors of response to immunotherapy [18, 20, 22, 24]. Therefore, either alone or in addition to PD-L1, TMB may improve patient selection for immunotherapy monotherapy or combination therapy [9, 18, 24].
Whole genome sequencing-based TMB has not received formal approval as a predictive biomarker for immunotherapy response neither in the USA [4] nor in Europe [1, 2]. Specific challenges that hamper implementation are a lack of sufficient evidence, standardization of TMB measurement [1], and the high costs for WGS. Therefore, the main objective of this study was to synthesize the available evidence on WGS-based TMB in a decision model and perform an early cost-effectiveness analysis of using WGS-based TMB as an immunotherapy predictive biomarker in treatment selection for patients with metNSCLC ineligible for targeted therapy, from a Dutch healthcare perspective. This study uses a previously developed decision model that simulates real-world Dutch patients with non-squamous metNSCLC [25].
2 Methods
2.1 Cost-Effectiveness Analysis
An early cost-effectiveness analysis was performed using a previously developed diagnostic-treatment microsimulation model for patients with non-squamous metNSCLC in the Netherlands [25]. The model was adapted to simulate different strategies for selecting patients for first-line immunotherapy with or without ChT based on PD-L1 expression and/or TMB.
Costs in Euros were included from a Dutch healthcare perspective and indexed to calendar year 2022. The healthcare perspective was deemed sufficient because metNSCLC is a severe disease and is more prevalent in the elderly population (aged > 60 years) [26, 27]. Therefore, for this population, healthcare costs are the key cost component while non-medical costs such as productivity loss are less relevant in an economic evaluation [27]. Effects were measured in life-years (LYs) and quality-adjusted life-years (QALYs). Both cost and effects were measured from the start of first-line treatment to death; that is, a life time horizon was used [28]. Future cost and effects were discounted at 4.0% and 1.5%, respectively [28]. Cost and effects were translated to a net monetary benefit (NMB), with a willingness-to-pay (WTP) threshold of €80,000 per QALY, as recommended for this disease severity in the Netherlands [29]. A higher NMB implies that a strategy is more cost effective compared with a strategy with a lower NMB [30]. Strategies were also plotted on a cost-effectiveness plane and the efficiency frontier was determined, that is, the line connecting those strategies that provide the largest incremental gain in QALYs per Euro spent. For these efficient strategies, the incremental cost-effectiveness ratio (ICER) relative to the next less efficient strategy was calculated by dividing the incremental costs in Euros by the incremental gain in QALYs. Below, the decision model, strategies, input parameters, and output are described in more detail.
2.2 Description of the Model
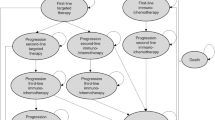
The decision model was developed as a discrete event simulation based on real-world data of patients with non-squamous metNSCLC in the Netherlands [31] and was described in detail elsewhere [25]. In brief, the decision model simulates a Dutch population of patients with non-squamous metNSCLC from diagnosis to death. Patients can have up to three lines of systemic treatment (first line, second line, and third line). At each treatment line, patients can move to a subsequent treatment line (from first line to second line and from second line to third line) or die. Patients can die from the disease or due to other causes. Death due to other causes was added as a background mortality competing risk and described using an age-specific and sex-specific life table of a general Dutch population adjusted for smoking [32]. The time to a subsequent treatment line or death due to disease is estimated by treatment line-specific parametric hazard functions. These parametric hazard functions are based on real-world data of patients treated with multiple lines of ChT [31]. See Sect. 1.1 and Tables S1 and S2 of the Electronic Supplementary Material (ESM).
In addition, the decision model is supplemented with a molecular diagnostic and treatment decision tree (Fig. S1b of the ESM) that allows changing patients’ treatment from ChT to targeted therapy or immunotherapy based on outcomes of molecular predictive biomarkers. For this study, we focus on the group of simulated patients that are ineligible for targeted treatment. In this group, first-line immunotherapy is simulated with or without additional ChT, based on molecular diagnostic strategies as described in Sect. 2.3. The adaptation of the decision model to simulate outcomes under first-line immunotherapy with or without ChT is described in Sect. 2.4.
2.3 Population and Strategies
The study population consists of patients who are non-squamous metNSCLC ineligible for targeted therapy, without contraindication nor refusal to immunotherapy. The patient population included those without a known targetable molecular biomarker (such as patients without epidermal growth factor receptor, anaplastic lymphoma kinase, ROS prot-oncogene 1, B-Raf proto-oncogene, MET [exon 14 skipping], and neurotrophic tropomyosin receptor kinase) [1,2,3]. According to the Dutch clinical guideline [1,2,3], immunotherapy (i.e., pembrolizumab monotherapy or pembrolizumab with ChT) is a recommended first-line therapy in this group of patients. The choice of monotherapy versus combination therapy depends on the results of immuno-biomarker test results.
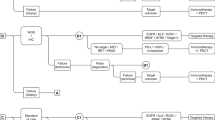
In the base-case analysis, six molecular biomarker testing and treatment strategies were evaluated. Strategies were defined by whether immuno-biomarker test included PD-L1 expression, TMB, or both PD-L1 expression and TMB (Fig. 1). Whole-genome sequencing and immunohistochemistry are assumed to have perfect sensitivity and specificity for TMB and PD-L1 testing, respectively. The prevalences of PD-L1 and TMB are given in Table 1 and they are assumed to be independent [18, 20]. Strategies are defined below.
Test and treat strategies A to F in the cost-effectiveness evaluation. Biom biomarker, ChT platinum-based chemotherapy doublets (here assumed cisplatin with pemetrexed), DB disease burden, IHC immunohistochemistry, Nsq-metNSCLC non-squamous advanced (inoperable/metastasis) non-small cell lung cancer ineligible for local treatment, pembro prembrolizumab, PD-L1 programmed death-ligand 1, SoC standard of care, Targ targeted, TMB tumor mutational burden, WGS whole-genome sequencing,  indicates the subgroup of patients excluded in the evaluation, i.e., patients with a known targetable biomarker and eligible for targeted therapies. + DB means that the treatment strategy is adapted as follows; first-line treatment of patients with high DB is changed from pembro to pembro + ChT. High DB is defined as those patients with a fast growing tumor, high number of metastasis, and/or weight loss [33] who are expected to have progressive disease within 3 months after start pembro.
indicates the subgroup of patients excluded in the evaluation, i.e., patients with a known targetable biomarker and eligible for targeted therapies. + DB means that the treatment strategy is adapted as follows; first-line treatment of patients with high DB is changed from pembro to pembro + ChT. High DB is defined as those patients with a fast growing tumor, high number of metastasis, and/or weight loss [33] who are expected to have progressive disease within 3 months after start pembro.
2.3.1 Test and Treat Strategy A: PD-L1 Alone
Programmed death-ligand 1 expression classifies patients into three subgroups; PD-L1 ≥ 50% (PD-L1 high), PD-L1 1–49% (PD-L1 intermediate), and PD-L1 < 1% (PD-L1 low) [13]. In strategy A, patients with PD-L1 high receive pembrolizumab monotherapy while those in the PD-L1-intermediate and PD-L1-low groups receive pembrolizumab with ChT (Fig. 1). Strategy A is aligned with the Dutch clinical guideline [1,2,3].
2.3.2 Test and Treat Strategy B: TMB Alone
In strategy B, treatment was assigned based on WGS-based TMB instead of PD-L1 expression. A threshold of 10 mut/Mb was used to classify patients into TMB-high and TMB-low groups [9, 22]. Patients with TMB ≥ 10 mut/Mb (TMB high) received pembrolizumab monotherapy, while patients with TMB < 10 mut/Mb (TMB low) received pembrolizumab with ChT (Fig 1).
2.3.3 Test and Treat Strategy C: PD-L1 and TMB
Strategy C combines PD-L1 expression and TMB level. Patients are classified into six subgroups based on PD-L1 expression and TMB level (i.e., PD-L1 high and TMB high, PD-L1 high and TMB low, PD-L1 intermediate and TMB high, PD-L1 intermediate and TMB low, PD-L1 low and TMB high, and PD-L1 low and TMB low). Patients with both PD-L1 high and TMB high receive pembrolizumab monotherapy, while patients with PD-L1 intermediate, PD-L1 low, or TMB low receive pembrolizumab with ChT (Fig. 1).
2.3.4 Test and Treat Strategy D: PD-L1 + DB
In a Dutch clinical practice, a proportion of patients with PD-L1 high are treated with pembrolizumab with ChT because of a high tumor/disease burden (high DB) [expert opinion JM and JA]. High DB is loosely defined as a fast-growing tumor, a high number of metastases, and/or weight loss [33]. The proportion of patients with high DB was assumed to be equal to the proportion of PD-L1-high patients with progressive disease within 3 months after the start of first-line pembrolizumab monotherapy (27%) [34]. In strategy D, the (randomly drawn) 27% of patients with PD-L1 high who have high DB are assumed to receive pembrolizumab with ChT (Fig. 1), while the remaining 73% of PD-L1-high patients with low DB receive pembrolizumab monotherapy. Patients with PD-L1 intermediate or PD-L1 low receive pembrolizumab with ChT as in strategy A.
2.3.5 Test and Treat Strategy E: TMB + DB
Strategy E is a modification of strategy B where the TMB-high group is split into two subgroups of high DB and low DB, as in strategy D. Of the TMB-high patients, 27% are assumed to have high DB and receive pembrolizumab with ChT (Fig. 1). The remaining 73% with low DB receive pembrolizumab monotherapy. Patients with TMB low receive pembrolizumab with ChT as in strategy B.
2.3.6 Test and Treat Strategy F: PD-L1 and TMB + DB
Strategy F is a modification of Strategy C, where the group of patients with PD-L1 high and TMB high is split into a high and low DB subgroup. The 27% with high DB receive pembrolizumab with ChT, while the remaining 73% receive pembrolizumab monotherapy. Remaining subgroups received pembrolizumab with ChT as in strategy C.
In all strategies, ChT was assumed to consist of cisplatin and pemetrexed. Irrespective of the strategy used, patients who progress to second-line treatment are assumed to receive cisplatin-pemetrexed and in the third line docetaxel if they had pembrolizumab monotherapy in the first line. Those who had pembrolizumab with ChT in the first line are assumed to receive docetaxel alone in the second line and gemcitabine in the third line.
2.4 Treatment Effectiveness
To simulate the progression-free and overall survival time under immunotherapy instead of under ChT, as in the originally discrete event simulation model (Fig. S1a of the ESM), the parametric hazard function for the event time from first-line treatment to second-line treatment and to death was adapted. A subgroup of patients has shown to have a long-term survival benefit (long-term survivors, LTS) after first-line pembrolizumab with or without ChT [35,36,37,38]. To account for the different survival of this subgroup, a mixture cure survival distribution was used to simulate the time to the event from first-line treatment to either second-line treatment or death [25, 39,40,41]. Under the mixture cure survival distribution, LTS were free from death due to non-small cell lung cancer but instead died due to other causes. Considering metNSCLC is a severe disease, the death due to other causes of LTS was described using an age-specific and sex-specific life table of a Dutch population of heavy smokers [32, 42, 43]. It was assumed that the size of the LTS subgroup is equal to the 5-year survival rate reported in keynote-001 [36] given PD-L1 expression, i.e., 29.7% for patients with PD-L1 ≥ 50%, 15.6% for patients with PD-L1 1–49% [36], and 0% in the PD-L1 < 1% subgroup. In contrast, non-LTS are subjected to disease progression (to second-line treatment or death) according to adjusted standard parametric hazard function. The parametric hazard function was adjusted by adding the hazard ratio for progression-free survival (PFS-HR) of pembrolizumab (with or without ChT) compared to ChT. The PFS-HR for non-LTS had to be calibrated such that, the PFS-HR total simulated patients (LTS plus non-LTS) given PD-L1 and TMB is equal to the derived hazard rate in Table 2. The PFS-HR in Table 2 depends on both a patient’s PD-L1 and TMB. The PFS-HRs for pembrolizumab monotherapy or pembrolizumab with ChT given PD-L1 were taken from published randomized controlled trials [12, 13, 34]. Likewise, the PFS-HRs given TMB was taken from a published randomized controlled trial [21]. See Sect. 1.2 of the ESM for more details regarding derivation of the PFS-HR in Table 2 and calibration of the PFS-HR for non-LTS. There was no adjustment made on parametric hazard functions of second-line and third-line treatments because patients who progressed to subsequent treatment lines received ChT (double or single agent) as described in Sect. 2.3.
2.5 Model Outcomes: LYs, QALYs, and Total Costs
Life-years were computed from the start of first-line treatment to death. For every strategy, the mean LYs lived per patient were aggregated as a weighted average of the mean LYs lived in each subgroup of the patient population stratified by PD-L1 expression, TMB level, and first-line treatment (Table 2). To compute QALYs, we multiplied LYs with health utilities during first line (progression free), second line (first-line progression), and third line (second-line progression). Health utilities were taken from literature [44] and assumed to be independent of treatment (Table 1). However, the influence of a specific treatment on a health utility was incorporated by using dis-utilities due to severe adverse events (SAEs) associated with each treatment regimen. SAEs were assumed to appear and dis-appear within the first month of treatment initiation after adequate SAEs management. All SAEs parameters were taken from the literature [35, 37, 45,46,47,48,49,50,51,52,53,54] and are listed in Table S3 of the ESM.
Total costs per patient per strategy consist of healthcare costs consumed from first-line treatment to death, including costs for drugs acquisition, drug administration, molecular biomarker testing (including standard diagnostic testing and WGS, see Sect. 1.3.1 of the ESM), routine laboratory tests, routine tumor assessment, managing SAEs, and end-of-life costs (Table 1 and Table S3 of the ESM). Cost were based on the Dutch costing manual [28], the Dutch Health Care Institute [55], and the published literature (Table 1 and Table S3 of the ESM). Details are given in the Sect. 1.3 of the ESM.
2.6 Model Validation
The model structure, simulated survival curves, and model assumptions were checked for face validity by consulting clinical oncology experts (JA, JM, and HG). In addition to that, the model was assessed using the Assessment of the Validation Status of Health-Economic decision Model checklist [61] by one of the authors (MS) [Sect. 3 of the ESM].
2.7 Scenario Analyses
We performed the following scenario analyses:
-
1.
Increased the proportion of patients with high DB from 27 to 50% (50% high DB).
-
2.
Withheld immunotherapy for patients with both PD-L1 low and TMB low, instead these patients were treated with ChT only (withholding immunotherapy).
-
3.
No LTS subgroup.
For further information, see Sect. 1.4 of the ESM.
2.8 Threshold Analyses
One-way, two-way, and three-way threshold analyses were performed for the prevalence of TMB high, the cost of pembrolizumab acquisition, pemetrexed acquisition, and WGS testing. Details are provided in Sect. 1.5 of the ESM.
2.9 Exploratory Analysis of the Impact of Adjusting the Predictive Value of TMB on the Treatment Effect of Pembrolizumab (With/Without ChT)
We explore the consequence of improving the predictive value of TMB for treatment benefit of pembrolizumab (with or without ChT). The predictive value of TMB was varied by increasing the treatment benefit in the responders’ group (i.e., TMB high) and decreasing the benefit in the non-responders’ group (i.e., TMB low). The magnitude of increase was 0.25 years to 1.75 years, in steps of 0.25 years. Further details are provided in Sect. 1.6 of the ESM.
3 Results
3.1 Base-Case Analysis
Costs and effects of the base-case analysis are given in Table 3 in order of increasing LYs. Strategy B was the least costly and also the least effective strategy. Discounted LYs (QALYs) per patients ranged from 2.61 (1.84) for strategy B (TMB alone) to 2.83 (2.00) for strategy F (PD-L1 and TMB + DB). Likewise, discounted lifetime costs per patient ranged from €120,800 for strategy B to €140,400 for strategy F. Strategy A (PD-L1 alone) had the highest NMB (€27,300) and F had the lowest NMB (€19,500). Strategy C (PD-L1 and TMB) and E (TMB + DB) were extendedly dominated, while strategies B, A, D (PD-L1 + DB), and F were on the cost-effectiveness frontier (Fig. 2a). The ICER for A compared to B was €74,900/QALY. For strategies D and F, the ICERs were 199,800/QALY and 299,200/QALY compared to the nearest less effective strategy on the frontier, respectively. Note that only strategy A has an ICER below the current guidelines with a WTP threshold of €80,000/QALY, i.e., strategy A was the optimal strategy.
Cost-effectiveness plane for the base-case analysis (a) and scenarios (b)–(d). b Fifty percent high disease burden (DB). c Withholding immunotherapy. d No long-term survivors subgroup. The black line connects strategies in a cost-effectiveness frontier. PD-L1 programmed death ligand 1, QALYs quality-adjusted life-years, TMB tumor mutational burden. *Range of the horizontal axis (discounted QALYs) is different from the range used in panels a–c
All strategies had total healthcare costs of more than €120,000 per patient. These high costs were mainly owing to drug acquisition costs (≥84%). Meanwhile, molecular biomarker diagnostics contributed 3% or less, even in strategies including WGS.
3.2 Scenario Analysis
When we assumed that the proportion of patients with high DB was 50% instead of 27% (Fig. 2b and Table S6b of the ESM), LYs (QALYs) and costs increased for strategies D, E, and F, while their NMBs decreased (Table S5b of the ESM). However, the order of strategies on the cost-effectiveness plane and the shape of the cost-effectiveness frontier (Fig. 2b) remained similar to that of the base-case analysis (Fig. 2a).
In the scenario of withholding immunotherapy for patients with PD-L1 low and TMB low (Fig. 2c and Table S6c of the ESM), a decrease in LYs (QALYs) and costs was observed for strategies C and F that include both PD-L1 expression and TMB in treatment selection. Because of a relative steep decrease in costs compared with the decrease in LYs (QALYs), strategy C now had the lowest costs and the highest NMB (€40,600) followed by F (€39,900), as can be seen from Fig. 2c. Strategies B, E, and A were dominated. The ICER of strategy F compared to C was €188,200/QALY and D compared to F was €727,400/QALY, much higher than the threshold of €80,000/QALY.
When we assumed no LTS subgroup for patients receiving immunotherapy, LYs (QALYs) were lower, while costs remained similar to the base-case costs. The order of strategies and the shape of the cost-effectiveness frontier (Fig. 2d and Table S6d of the ESM) remained similar to that of the base-case analysis. However, the incremental NMB of strategy A compared to strategy B decreased from €600 (Table 3) to €−30 (Table S6d of the ESM) given the WTP of €80,000/QALY. The ICER of strategy A compared to B was €80,300/QALY, close to the WTP threshold. Therefore, under assumption of no LTS, strategies A and B had comparable cost effectiveness at this WTP. Meanwhile, the ICER of strategy D compared to A and strategy F compared to D remained above the WTP threshold (Fig. 2d).
3.3 Threshold Analyses
Figure S2 of the ESM shows the results of the one-way threshold analyses. The NMB (at a WTP of €80,000/QALY) of each strategy (y-axis) is projected against the prevalence of TMB high (a), the cost of WGS testing (b), the cost of pembrolizumab acquisition (c), and the cost of pemetrexed acquisition (d). Strategy B had the highest NMB when the prevalence of TMB high is 40% or more (Fig. S2a of the ESM) and when WGS testing cost is €2500 or less (Fig. S2b of the ESM). In all other one-way threshold analyses, strategy A had the highest NMB. The ordering of strategies on the basis of NMB was insensitive to the cost of pembrolizumab acquisition (Fig. S2c of the ESM) and pemetrexed acquisition (Fig. S2d of the ESM), with strategy A consistently having the highest NMB.
Figure 3 shows the ranking of strategies A to F with respect to NMB in a two-way threshold analysis varying the costs of pembrolizumab and WGS testing. Strategy A had the highest NMB over most of the cost range evaluated for pembrolizumab and WGS testing, followed by strategy D with the second highest NMB. Strategy B had highest NMB only for costs of pembrolizumab above €4000 and WGS testing below €2500. None of the other strategies achieved the highest NMB over the range of costs of pembrolizumab acquisition and WGS testing evaluated.
Ranking of the strategies with respect to the net monetary benefit (NMB) in a two-way threshold analysis varying the costs of pembrolizumab acquisition and whole-genome sequencing (WGS) testing. DB disease burden, PD-L1 programmed death-ligand 1, TMB tumor mutational burden. The costs were decreased from the base-case values to €500 and €250 in steps of €500 and €250 for pembrolizumab acquisition and WGS testing, respectively. Net monetary benefit was computed using a willingness-to-pay threshold of €80,000/QALY. For example, at their base-case values, that is at a cost of pembrolizumab acquisition of €5721 and WGS testing of €3305, strategy A: PD-L1 alone had the highest NMB, followed by B: TMB alone, D: PD-L1 + DB, E: TMB + DB, C: PD-L1 and TMB, and last, F: PD-L1 and TMB + DB. The strategy in a higher rank is regarded as cost effective compared with strategies in a lower rank
Results of jointly varying the costs of pemetrexed acquisition and WGS testing (Fig. S3 of the ESM) and the costs of pembrolizumab acquisition and pemetrexed acquisition (Fig. S4 of the ESM) mostly confirm results from the one-way threshold analyses. The optimal strategy is most sensitive to costs for WGS testing, with strategy B replacing Strategy A as the optimal strategy when the costs for WGS testing decreased. The exact extent to which WGS testing costs should be lowered for a change in the optimal strategy depends on the cost of pemetrexed acquisition, with stronger decreases in WGS testing costs required at lower pemetrexed acquisition costs (Fig. S3 of the ESM). Strategy D: PD-L1 + DB became the optimal strategy when pembrolizumab acquisition costs were €3000 or less and pemetrexed acquisition costs were €500 or less. Otherwise, strategy A remains the optimal strategy (Fig. S4 of the ESM). Results of the three-way threshold analysis (Fig. S5 of the ESM) shows that strategies of combining PD-L1 and TMB testing (i.e., strategies C and F) had the highest NMB when the cost of both WGS testing, pembrolizumab acquisition, and pemetrexed acquisition were simultaneously decreased by at least 47%, 39%, and 43%, respectively.
3.4 Exploratory Analysis on Improving the Predictive Value of TMB
In the base-case analysis, the difference in mean undiscounted LYs during first-line treatment between the TMB-high and TMB-low subgroups was 0.329 years for pembrolizumab and 0.544 years for pembrolizumab with ChT. When the difference in LYs between TMB high and TMB low increased by 0.25 years, the NMB of strategy B was approximately equal to the NMB of strategy A (Fig. S6 of the ESM). At an increase of more than 0.25 years, strategy B replaced strategy A as the optimal strategy in term of NMB (Fig. S6 of the ESM). However, the shape of the cost-effectiveness frontier remained similar to that of the base-case analysis with strategy B having the lowest QALYs (LYs) and costs, and strategy F having highest QALYs (LYs) and costs (Fig. S7 of the ESM). Despite the increase in the difference in LYs between the TMB-high and TMB-low subgroups, the other TMB testing strategies (C, E, and F) remained not cost-effective.
4 Discussion
This study reports on an early cost-effectiveness analysis of using WGS-based TMB for treatment selection compared to using PD-L1 expression in patients with non-squamous metNSCLC ineligible for targeted therapy [1,2,3,4]. Six test and treatment strategies based on TMB level and/or PD-L1 expression were simulated. Treatment selection based on WGS-based TMB alone was the least costly and least effective strategy under the base-case assumptions, with patients with TMB ≥ 10 mut/Mb receiving pembrolizumab monotherapy and patients with TMB < 10 mut/Mb receiving pembrolizumab with ChT. Compared with the TMB-alone strategy, the current strategy where PD-L1 alone guides treatment selection (patients with PD-L1 ≥ 50% receiving pembrolizumab monotherapy and those with PD-L1 < 50% receiving pembrolizumab with ChT) was cost effective with an ICER of €74,900/QALY and an incremental NMB of €600 at a WTP of €80,000/QALY.
The strategy of using TMB alone replaced PD-L1 alone as an optimal strategy (cost effective with highest NMB compared with other strategies) when the prevalence of TMB high was ≥ 40%, the predictive value of TMB on immunotherapy benefit increased by ≥ 0.5 years, or the cost of WGS testing reduced by ≤ 24%. Two-way threshold analyses shows that the extent to which WGS testing costs should be reduced to turn TMB alone cost effective depends on pembrolizumab and pemetrexed acquisition cost, with a higher reduction of WGS testing cost required at decreased costs of pembrolizumab or pemetrexed acquisition.
A strategy of combining PD-L1 and TMB testing had the highest NMB only when the cost of WGS testing, of pembrolizumab acquisition, and of pemetrexed acquisition were simultaneously decreased by at least 47%, 39%, and 43%, respectively. Alternatively, the strategy of combined PD-L1 and TMB testing would result in the highest NMB if patients with the least benefit of pembrolizumab, that is, patients with both PD-L1 < 1% and TMB < 10 mut/Mb (30% of all patients) were treated with ChT alone, thus withholding immunotherapy in this group. A scenario of withholding immunotherapy resulted in a saving of €14,300 per patient with a loss in QALYs (LYs) of 0.02 (0.02) per patient, compared with the current strategy of using PD-L1 alone.
Only one previous study, the study by Li et al. [62], also investigated the value of blood TMB testing versus PD-L1 testing for immunotherapy selection, but their study was based on a population of previously treated patients with advanced non-small cell lung cancer. Apart from the specific population, our model differs from theirs with respect to model structure, data used for model quantification, test and treatment strategies simulated, and country perspective (China/USA vs the Netherlands). However, on a global level, our results are similar to those reported by Li et al. Taking a Chinese and US healthcare perspective, the authors reported that testing for blood TMB to select atezolizumab (immunotherapy) or docetaxel (chemotherapy) resulted in lower costs and lower health effects compared with selection based on PD-L1 expression; however, it was cost effective under the Chinese and the US jurisdiction [62]. Li et al. did not consider the cost effectiveness of using the combination of PD-L1 and blood TMB testing [62].
A strength of our study is the fact that we used a previously developed, validated, and published decision analytic model [25]. This decision model reflects the Dutch clinical setting and it is based on real-world patient data from Dutch hospitals [31]. The analysis accounts for outcome variation at the patient level, as the microsimulation framework used in this analysis incorporates patient heterogeneity by including characteristics such as performance status, age, and patient’s treatment history [63].
Furthermore, through scenario and threshold analyses, the influence of important clinical aspects on the conclusions of the study were investigated such as the predictive value of TMB, drug acquisition costs, withholding immunotherapy, and accounting for a patient’s disease condition in treatment decisions. Thus, the findings of this study contribute to the wider range of aspects that researchers and decision makers may take into consideration to optimize immunotherapy treatment decisions for patients with metNSCLC.
Availability of high-quality evidence is a recognized challenge for an early health technology assessment [64]. Our study utilizes the available evidence as much as possible. In the absence of strong evidence, plausible assumptions were made in consultation with clinical experts. All assumptions and parameter derivations are extensively described in the ESM and were validated by clinical experts. Important model assumptions and study limitations are as follows:
First, to the best of our knowledge, there are no published randomized controlled trial data reporting the PFS-HR of pembrolizumab (with/without) ChT compared to ChT as a function of both PD-L1 expression and TMB level. Therefore, to simulate patients’ LYs lived during first line treatment in subgroups defined by both PD-L1 and TMB level, the PFS-HRs of pembrolizumab (with/without) ChT compared to ChT as a function of both PD-L1 and TMB level were derived mathematically. Indirect comparison shows that the derived marginal PFS-HR of pembrolizumab with ChT compared to ChT given the TMB level were comparable to recently published values from analysis of keynote-189 [65]. The derived PFS-HRs were 0.43 in the TMB-high subgroup and 0.67 in TMB-low subgroup. These are reasonably close to the published PFS-HRs of 0.32 (95% CI 0.21–0.51) for TMB high and 0.51 (95% CI 0.35–0.74) for TMB low [65].
Second, a mixture cure survival distribution was used to account for the presence of a subgroup of patients with long-term survival benefit after pembrolizumab [35,36,37]. Whether the improvement in long-term survival after first-line pembrolizumab as observed in randomized controlled trials is sufficient to assume the presence of such a subgroup with a life-time durable benefit (cured) is uncertain and has yet to be validated. Therefore, we repeated the analysis assuming no such LTS subgroup (no-LTS). Compared with the base-case results, assuming no LTS resulted in similar costs although QALYs decreased for all strategies. Consequently, the NMB of PD-L1 alone was approximately equal to the NMB of TMB alone with an incremental NMB of strategy A compared with strategy B of €− 30. The ordering of strategies and cost-effectiveness outcomes of other strategies (C, D, E, and F) remained comparable to the base-case analysis.
Third, although TMB has been shown to have predictive value for different immunotherapies such as nivolumab and ipilimumab, our study is restricted to the evaluation of TMB testing for the selection of patients for pembrolizumab with or without ChT as first-line treatment option in advanced non-small cell lung cancer. In the Netherlands, pembrolizumab is a recommended immunotherapy regimen [3] and the most used as a first-line treatment in this subgroup of patients [66]. In addition to that, the study simulated only non-squamous metNSCLC and did not include squamous cell metNSCLC. About 30% of non-small cell lung cancers have squamous cell histology [67], which leads to different clinical management than for non-squamous histology [1, 2, 4]. Therefore, the reported findings may have limited generalizability to patients with squamous metNSCLC and to healthcare settings where other immunotherapies such as nivolumab or ipilimumab are used.
Fourth, this evaluation was performed assuming perfect sensitivity and specificity of IHC for PD-L1 testing and WGS for TMB testing. Whole genome sequencing is considered as a golden standard test for TMB [18, 19], with presumably near-perfect sensitivity and specificity. For this analysis, the assumption of perfect sensitivity and specificity was extended to IHC for PD-L1 testing. Some misclassification with respect to PD-L1 expression will lead to somewhat less favorable cost-effectiveness outcomes for the PD-L1-based strategies but is unlikely to change our main cost-effectiveness result that a TMB treatment strategy leads to the highest NMB, albeit a lower health outcome than a PD-L1-alone strategy.
Fifth, the study did not take into account a possible effect on the survival of a delay in treatment initiation caused by the waiting time for molecular diagnostic test results [43]. To what extent the inclusion of a delay in treatment initiation in the model would have affected the results is not immediately clear. Although the standard-of-care diagnostic strategy has a shorter average waiting time (in term of turnaround time) compared with WGS, i.e., 8 [5] versus 10 working days [68], respectively, both are within an acceptable maximum turnaround time of 2 weeks [69, 70]. This suggests the impact would be small. In addition, note that WGS has the advantage of allowing most (all) molecular markers (except PD-L1) to be tested once together, which is likely to result in a low tumor exhaustion and an increase in the proportion of patients receiving correct therapy within a maximum turnaround time [68, 70].
Sixth, it was not feasible to perform a probabilistic sensitivity analysis because of the computational run time. Instead, one-way and multi-way threshold analyses were performed for key variables such as test and drug acquisition costs and the predictive value of TMB on treatment benefit.
Finally, this study evaluated the health economic utility of using TMB as a WGS-based biomarker for immunotherapy treatment selection. However, WGS has a wider application both in cancer and in other indications, such as rare diseases. Evaluation of the economic utility of WGS for other indications showed that WGS could also be cost effective when used as the preferred front-line diagnostic tool for a wider patient population and WGS testing costs are reduced [11, 71,72,73].
5 Conclusions
Given the current evidence, the use of WGS-based TMB is not cost effective in immunotherapy treatment selection in metNSCLC compared to using PD-L1 alone. Whole genome sequencing-based TMB testing could be a cost-effective strategy in therapy selection when there is a reduction in the cost of WGS testing. Alternatively, a combination of WGS-based TMB and PD-L1 testing could become a cost-effective strategy (with a substantial cost saving) if patients with both TMB <10 mut/mB and PD-L1 <1% are treated with ChT alone, thus withholding immunotherapy in this group. It is recommended to further investigate the possibility of limiting immunotherapy in patients who have a low likelihood of benefiting from this treatment.
References
Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):358–76.
Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–57.
Federatie Medisch Specialisten. Non-small cell lung carcinoma [in Dutch: Niet kleincellig longcarcinoom]. 2023.
National Comprehensive Cancer Network. Non-small cell lung cancer (version 3.2023). 2023. https://www.nccn.org/guidelines/category_1. Accessed 3 Oct 2023.
Steeghs EMP, Groen HJM, Schuuring E, Aarts MJ, Damhuis RAM, Voorham QJM, et al. Mutation-tailored treatment selection in non-small cell lung cancer patients in daily clinical practice. Lung Cancer. 2022;167:87–97.
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–66.
Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5): e192535.
Greillier L, Tomasini P, Barlesi F. The clinical utility of tumor mutational burden in non-small cell lung cancer. Transl Lung Cancer Res. 2018;7(6):639–46.
Fumet JD, Truntzer C, Yarchoan M, Ghiringhelli F. Tumour mutational burden as a biomarker for immunotherapy: current data and emerging concepts. Eur J Cancer. 2020;131:40–50.
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–8.
Simons MJHG, Retel VP, Ramaekers BLT, Butter R, Mankor JM, Paats MS, et al. Early cost effectiveness of whole-genome sequencing as a clinical diagnostic test for patients with inoperable stage IIIB, C/IV non-squamous non-small-cell lung cancer. Pharmacoeconomics. 2021;39(12):1429–42.
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92.
US Food and Drug Administration. PD-L1 IHC 22C3 PHARMDX [premarket approval]. Immunohistochemistry assay, antibody, programmed death-ligand 1. 2015.
US Food and Drug Administration. Pembrolizumab (Keytruda) checkpoint inhibitor. 2016.
European Medicines Agency [EMA]. Keytruda. Pembrolizumab. 2016.
Merino DM, McShane LM, Fabrizio D, Funari V, Chen SJ, White JR, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8(1):e000147.
Mankor JM, Paats MS, Groenendijk FH, Roepman P, Dinjens WNM, Dubbink HJ, et al. Impact of panel design and cut-off on tumour mutational burden assessment in metastatic solid tumour samples. Br J Cancer. 2020;122(7):953–6.
Sutherland KD, Vissers JHA. Balancing the count: harmonizing panel-based tumor mutational burden assessment. J Thorac Oncol. 2020;15(7):1106–9.
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26.
Galvano A, Gristina V, Malapelle U, Pisapia P, Pepe F, Barraco N, et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): a systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2021;6(3): 100124.
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson G, Audigier-Valette C, et al. Nivolumab (nivo) plus ipilimumab (ipi) vs platinum-doublet chemotherapy (PT-DC) as first-line (1L) treatment (tx) for advanced non-small cell lung cancer (NSCLC): initial results from CheckMate 227. Cancer Res. 2018;78(13):CT077.
Meng G, Liu X, Ma T, Lv D, Sun G. Predictive value of tumor mutational burden for immunotherapy in non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2022;17(2): e0263629.
Kao C, Powers E, Wu Y, Datto MB, Green MF, Strickler JH, et al. Predictive value of combining biomarkers for clinical outcomes in advanced non-small cell lung cancer patients receiving immune checkpoint inhibitors. Clin Lung Cancer. 2021;22(6):500–9.
Mfumbilwa ZA, Wilschut JA, Simons M, Ramaekers B, Joore M, Retel V, et al. Development and validation of a decision model for the evaluation of novel lung cancer treatments in the Netherlands. Sci Rep. 2023;13(1):2349.
Netherlands comprehensive cancer organisation [IKNL]. Prevalence by year, 5 year prevalence: non small-cell lung carcinoma. 2023. https://iknl.nl/en/ncr/ncr-data-figures. Accessed 6 Nov 2023.
Zorginstituut Nederland [ZIN]. Package advice pembrolizumab (Keytruda®) [in Dutch: Pakketadvies pembrolizumab (Keytruda®)]. Zorginstituut Nederland. 2016. Dec 14.
Kanters TA, Bouwmans CAM, van der Linden N, Tan SS, Hakkaart-van RL. Update of the Dutch manual for costing studies in health care. PLoS One. 2017;12(11): e0187477.
Vijgen S, van Heesch F, Obradovic M. Ziektelast in de praktijk; de theorie en praktijk van het berekenen van ziektelast bij pakketbeoordelingen. Zorginstituut Nederland. 2018.
Paulden M. Calculating and Interpreting ICERs and Net benefit. Pharmacoeconomics. 2020;38(8):785–807.
Cramer-vander Welle CM, Peters BJM, Schramel F, Klungel OH, Groen HJM, van de Garde EMW, et al. Systematic evaluation of the efficacy-effectiveness gap of systemic treatments in metastatic nonsmall cell lung cancer. Eur Respir J. 2018;52(6):1801101.
Wolff HB, Alberts L, van der Linden N, Bongers ML, Verstegen NE, Lagerwaard FJ, et al. Cost-effectiveness of stereotactic body radiation therapy versus video assisted thoracic surgery in medically operable stage I non-small cell lung cancer: a modeling study. Lung Cancer. 2020;141:89–96.
Higuera Gomez O, Moreno Paul A, Ortega Granados AL, Ros Martinez S, Perez Parente D, Ruiz Gracia P, et al. “High tumor burden” in metastatic non-small cell lung cancer: defining the concept. Cancer Manag Res. 2021;13:4665–70.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Gadgeel S, Rodriguez-Abreu D, Speranza G, Esteban E, Felip E, Domine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–17.
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–27.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score >/= 50. J Clin Oncol. 2021;39(21):2339–49.
Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Domine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 Study. J Clin Oncol. 2023. https://doi.org/10.1200/JCO.22.01989.
Bullement A, Latimer NR, Bell GH. Survival extrapolation in cancer immunotherapy: a validation-based case study. Value Health. 2019;22(3):276–83.
Othus M, Bansal A, Koepl L, Wagner S, Ramsey S. Accounting for cured patients in cost-effectiveness analysis. Value Health. 2017;20(4):705–9.
Ouwens M, Mukhopadhyay P, Zhang Y, Huang M, Latimer N, Briggs A. Estimating lifetime benefits associated with immuno-oncology therapies: challenges and approaches for overall survival extrapolations. Pharmacoeconomics. 2019;37(9):1129–38.
Statistics Netherlands (CBS). Heavy smokers cut their lifespan by 13 years on average. 2017. https://www.cbs.nl/en-gb/news/2017/37/heavy-smokers-cut-their-lifespan-by-13-years-on-average. Accessed 18 May 2022.
Vellekoop H, Huygens S, Versteegh M, Szilberhorn L, Zelei T, Nagy B, et al. Guidance for the harmonisation and improvement of economic evaluations of personalised medicine. Pharmacoeconomics. 2021;39(7):771–88.
Chouaid C, Agulnik J, Goker E, Herder GJ, Lester JF, Vansteenkiste J, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8(8):997–1003.
He X, Wang J, Li Y. Efficacy and safety of docetaxel for advanced non-small-cell lung cancer: a meta-analysis of phase III randomized controlled trials. Onco Targets Ther. 2015;8:2023–31.
Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: a meta-analysis. Lung Cancer. 2019;135:196–204.
Holleman MS, Al MJ, Zaim R, Groen HJM, Uyl-de Groot CA. Cost-effectiveness analysis of the first-line EGFR-TKIs in patients with non-small cell lung cancer harbouring EGFR mutations. Eur J Health Econ. 2020;21(1):153–64.
Banz K, Bischoff H, Brunner M, Chouaid C, de Castro CJ, de Marinis F, et al. Comparison of treatment costs of grade 3/4 adverse events associated with erlotinib or pemetrexed maintenance therapy for patients with advanced non-small-cell lung cancer (NSCLC) in Germany, France, Italy, and Spain. Lung Cancer. 2011;74(3):529–34.
Chouaid C, Loirat D, Clay E, Millier A, Godard C, Fannan A, et al. Cost analysis of adverse events associated with non-small cell lung cancer management in France. Clinicoecon Outcomes Res. 2017;9:443–9.
Campone M, Yang H, Faust E, Kageleiry A, Signorovitch JE, Zhang J, Gao H. Cost of adverse events during treatment with everolimus plus exemestane or single-agent chemotherapy in patients with advanced breast cancer in Western Europe. J Med Econ. 2014;17(12):837–45.
Paracha N, Abdulla A, MacGilchrist KS. Systematic review of health state utility values in metastatic non-small cell lung cancer with a focus on previously treated patients. Health Qual Life Outcomes. 2018;16(1):179.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–90.
Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK, Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based C. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol. 2010;5(2):260–74.
Zorginstituut Nederland [ZIN]. Drug cost [in Dutch: Medicijnkosten]. 2023. https://www.medicijnkosten.nl/. Accessed 3 Oct 2023.
Zorginstituut Nederland [ZIN]. Pharmacotherapeutic compass (in Dutch: Farmacotherapeutisch Kompas). 2023. https://www.farmacotherapeutischkompas.nl/. Accessed 3 Oct 2023.
Pasmans CTB, Tops BBJ, Steeghs EMP, Coupe VMH, Grunberg K, de Jong EK, et al. Micro-costing diagnostics in oncology: from single-gene testing to whole-genome sequencing. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):413–4.
van der Linden N, Bongers ML, Coupe VM, Smit EF, Groen HJ, Welling A, et al. Costs of non-small cell lung cancer in the Netherlands. Lung Cancer. 2016;91:79–88.
Schneider PP, Pouwels X, Passos VL, Ramaekers BLT, Geurts SME, Ibragimova KIE, et al. Variability of cost trajectories over the last year of life in patients with advanced breast cancer in the Netherlands. PLoS One. 2020;15(4): e0230909.
Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174–9.
Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61.
Li WQ, Li LY, Bai RL, Qian L, Chen NF, Cui JW. Cost-effectiveness of programmed cell death ligand 1 testing and tumor mutational burden testing of immune checkpoint inhibitors for advanced non-small cell lung cancer. Chin Med J (Engl). 2020;133(21):2630–2.
Davis S, Stevenson M, Tappenden P, Wailoo A. NICE DSU Technical Support Document 15: cost-effectiveness modelling using patient-level simulation. 04-2014.
Tummers M, Kvaerner K, Sampietro-Colom L, Siebert M, Krahn M, Melien O, et al. On the integration of early health technology assessment in the innovation process: reflections from five stakeholders. Int J Technol Assess Health Care. 2020;36(5):481–5.
Garassino MC, Gadgeel S, Novello S, Halmos B, Felip E, Speranza G, et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes with pembrolizumab plus chemotherapy versus chemotherapy for metastatic NSCLC. JTO Clin Res Rep. 2023;4(1): 100431.
Cramer-van der Welle CM, Verschueren MV, Tonn M, Peters BJM, Schramel F, Klungel OH, et al. Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep. 2021;11(1):6306.
Antoni van Leeuwenhoek. Non-small cell lung carcinoma. 2023. https://www.avl.nl/en/information-about-cancer/types-of-cancer/lung-cancer/non-small-cell-lung-carcinoma/. Accessed 6 Nov 2023.
Roepman P, de Bruijn E, van Lieshout S, Schoenmaker L, Boelens MC, Dubbink HJ, et al. Clinical validation of whole genome sequencing for cancer diagnostics. J Mol Diagn. 2021;23(7):816–33.
Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823–59.
Wolff HB, Steeghs EMP, Mfumbilwa ZA, Groen HJM, Adang EM, Willems SM, et al. Cost-effectiveness of parallel versus sequential testing of genetic aberrations for stage IV non-small-cell lung cancer in the Netherlands. JCO Precis Oncol. 2022;6: e2200201.
Simons MJHG, Uyl-de Groot CA, Retel VP, Mankor JM, Ramaekers BLT, Joore MA, et al. Cost-effectiveness and budget impact of future developments with whole-genome sequencing for patients with lung cancer. Value Health. 2022. https://doi.org/10.1016/j.jval.2022.07.006.
Stark Z, Schofield D, Alam K, Wilson W, Mupfeki N, Macciocca I, et al. Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet Med. 2017;19(8):867–74.
Rosenquist R, Cuppen E, Buettner R, Caldas C, Dreau H, Elemento O, et al. Clinical utility of whole-genome sequencing in precision oncology. Semin Cancer Biol. 2022;84:32–9.
Acknowledgements
The authors thank all members of the Technology Assessment of Next Generation Sequencing in Personalized Oncology (TANGO) consortium (https://zenodo.org/communities/tango-wgs/?page=1&size=20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the Netherlands Organization for Health Research and Development (ZonMw) [Grant number 846001002], the Dutch Cancer Society (KWF), and the Dutch healthcare insurance company Zilveren kruis Achmea. The funding sources had no involvement in the conduct of this research. The Open Access Fee was paid by the Department of Epidemiology and Data Science, Amsterdam University Medical Centers.
Conflict of interest
Joachim G.J.V. Aerts reports personal fees and non-financial support from MSD, personal fees from BMS, personal fees from Boehringer Ingelheim, personal fees from Amphera, personal fees from Eli-Lilly, personal fees from Takeda, personal fees from Bayer, personal fees from Roche, and personal fees from Astra Zeneca, outside the submitted work. In addition, Joachim G.J.V. Aerts has a patent allogenic tumor cell lysate licensed to Amphera, a patent combination immunotherapy in cancer pending, and a patent biomarker for immunotherapy pending. Zakile A. Mfumbilwa, Martijn J.H.G. Simons, Bram Ramaekers, Valesca P. Retèl, Joanne M. Mankor, Harry J.M. Groen, Manuela Joore, Janneke A. Wilschut, and Veerle M.H. Coupé have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
All methods were carried out in accordance with relevant guidelines and regulations. The study was performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this article and its supplementary materials.
Code availability
The codes for the decision model are available on request. Request should be addressed to the corresponding author.
Authors’ contributions
ZAM: study conception and design, methodology, performed the analysis, data interpretation, writing main manuscript, review and editing. JAW and VMHC: study conception and design, methodology, data interpretation, writing main manuscript, review, editing, and study supervision. MJHGS, BR, VPR, and MJ: study conception and design, methodology, data interpretation, writing review and editing. JMM and JGJVA: study conception, validation of model assumptions and data, data interpretation and giving clinical contents, writing review and editing. HJMG: validation of model assumptions and data, data interpretation and giving clinical contents, writing review and editing. All authors reviewed the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mfumbilwa, Z.A., Simons, M.J.H.G., Ramaekers, B. et al. Exploring the Cost Effectiveness of a Whole-Genome Sequencing-Based Biomarker for Treatment Selection in Patients with Advanced Lung Cancer Ineligible for Targeted Therapy. PharmacoEconomics 42, 419–434 (2024). https://doi.org/10.1007/s40273-023-01344-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01344-w