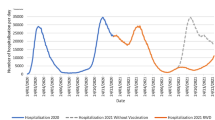
Abstract
Background and Objective
Coronavirus disease 2019 (COVID-19) vaccines are extremely effective in preventing severe disease, but their real-world cost effectiveness is still an open question. We present an analysis of the cost-effectiveness and economic impact of the initial phase of the COVID-19 vaccination rollout in the Basque Country, Spain.
Methods
To calculate costs and quality-adjusted life years for the entire population of the Basque Country, dynamic modelling and a real-world data analysis were combined. Data on COVID-19 infection outcomes (cases, hospitalisations, intensive care unit admissions and deaths) and population characteristics (age, sex, socioeconomic status and comorbidity) during the initial phase of the vaccination rollout, from January to June of 2021, were retrieved from the Basque Health Service database. The outcomes in the alternative scenario (without vaccination) were estimated with the dynamic model used to guide public health authority policies, from February to December 2020. Individual comorbidity-adjusted life expectancy and costs were estimated.
Results
By averting severe disease-related outcomes, COVID-19 vaccination resulted in monetary savings of €26.44 million for the first semester of 2021. The incremental cost-effectiveness ratio was €707/quality-adjusted life year considering official vaccine prices and dominant real prices. While the analysis by comorbidity showed that vaccines were considerably more cost effective in individuals with pre-existing health conditions, this benefit was lower in the low socioeconomic status group.
Conclusions
The incremental cost-effectiveness ratio of the vaccination programme justified the policy of prioritising high-comorbidity patients. The initial phase of COVID-19 vaccination was dominant from the perspective of the healthcare payer.
Similar content being viewed by others
Measurement of costs and benefits of coronavirus disease 2019 vaccines disaggregated by socioeconomic status and level of comorbidity has yet to be estimated. |
The initial phase of coronavirus disease 2019 vaccination rendered more health gains and saved costs. |
Though no clear differences were observed by socioeconomic status, vaccines were more cost effective in individuals with pre-existing health conditions. |
1 Introduction
The fast rollout of coronavirus disease 2019 (COVID-19) vaccination programmes was of paramount importance in containing the pandemic [1]. Although the licenced COVID-19 vaccines are extremely effective in preventing severe disease symptoms, their real-world cost utility is still an open question [2, 3]. From the macroeconomic perspective, externalities associated with the COVID-19 pandemic have produced substantial drops in gross domestic product and the economic benefit of vaccination programmes is overwhelmingly positive [4]. However, a microeconomic analysis in terms of the cost and effectiveness of these programmes is still ongoing [2].
Dynamic models have been recognised as a powerful tool for understanding the impact of vaccines on COVID-19-related outcomes worldwide: infections, hospitalisations, intensive care unit (ICU) admissions and deaths [5]. The benefits of vaccine administration depend, however, on individual characteristics such as sex, age, socioeconomic status (SES) and pre-existing health conditions (comorbidities) [6]. Even though some models have used age-stratified populations, the clinical characteristics of the individuals vaccinated are not considered in many dynamic models, possibly to avoid an exponential increase in model complexity [3]. This makes it difficult to fully understand the economic impact of COVID-19 vaccination programmes. Presently, it is well known that elderly and vulnerable individuals with comorbidities are at a higher risk of developing severe disease and therefore would be likely to benefit the most from vaccination [7]. In addition, social deprivation, on a continuous scale, is known to be a risk factor for death from COVID-19 [8, 9].
Real-world data from national registries contain individual information on diagnoses and treatments, and have been used to assess changes in social and clinical determinants of COVID-19 outcomes [6]. However, to our knowledge, while economic evaluations have been conducted to analyse hypothetical scenarios with various assumptions concerning the supply, cost and effectiveness of vaccines, real costs per quality-adjusted life-years of COVID-19 vaccination programmes have yet to be estimated [10,11,12].
Given this gap in the literature, we have used individual-level data from the Basque Health Service, disaggregated by SES and level of comorbidity, to calculate the real-world cost-utility and economic impact of COVID-19 vaccines administered to the population in the Basque Country, Spain. We present results obtained for the initial vaccination rollout phase, from January to June 2021.
2 Methods
2.1 Design
This study combined dynamic modelling and real-world data to calculate the cost effectiveness of the initial phase of the COVID-19 vaccination rollout in the Basque Country from the perspective of the healthcare payer, i.e. the Basque Health Service. Given the limitation of dynamic models based on differential equations to incorporate the clinical and sociodemographic characteristics of the population and enable a subgroup analysis, we analysed the health service data lake constructed on an Oracle Analytics System (OAS) to provide effectiveness and costs for such subgroup analysis. In this way, the dynamic model reproduced the behaviour of the COVID-19 epidemic in the scenario without vaccines jointly for the entire Basque population, but the results associated with COVID-19 could be disaggregated by combining them with the individual characteristics contained in the OAS. The OAS is a platform containing complete healthcare information (clinical and administrative datasets) about the entire population of the Basque Country (2.3 million people) in an anonymised format. It provided information on COVID-19 infections related to disease outcomes and the use of healthcare resources during the first semester of 2021. We estimated the outcomes for the scenario with no vaccination using the dynamic SHARUCD model (described in more detail below), developed to guide the decision making of the public health authorities in the Basque Country [13,14,15]. We then assessed population-level benefits from the vaccination programme by comparing the observed OAS data for 2021 (scenario with vaccination) with the disease outcomes predicted by the dynamic model (scenario with no vaccination). Specifically, we calculated the incremental cost-effectiveness ratio (ICER), by dividing the incremental cost (cost with vaccination minus cost without vaccination) by the incremental utility (utility with vaccination minus utility without vaccination) [2]. Note that we did not analyse several hypothetical sub-group delivery options. Instead, we evaluated the consequences for each subgroup of the actual rollout strategy compared with a no-vaccination strategy.
2.2 Vaccination Programme in the Basque Country
All the COVID-19 vaccines administered to the entire population in the Basque Country were delivered in a centralised manner by the Basque Health Service in phases by priority group, these groups including elderly people and individuals with comorbidities. By the end of June 2021, the percentages of fully vaccinated individuals by age group were 5% in 0- to 29-year-olds, 19% in 30- to-49-year-olds, 66% in 50- to 69-year-olds and 95% in ≥ 70-year-olds. Between January and June 2021, a total of 2,071,204 vaccine doses were administered in the Basque Country; of these, 70% were of the Pfizer-BioNTech vaccine, while 17% were Oxford-AstraZeneca, 10% were Moderna and 4% were Johnson & Johnson’s Janssen vaccines.
2.3 COVID-19 Infection Outcomes
The observed COVID-19 infection outcomes considered in the population, from January to June 2021, were the total number of detected cases of infection, hospitalisations, ICU admissions and deaths in the scenario with vaccination and these were obtained from the OAS [6]. The challenge was to estimate, by modelling, the outcomes in the alternative scenario with no vaccination in the same population. For this, we used the working dynamic SHARUCD model to estimate the number of cases of each one of the aforementioned outcomes. This model was validated with empirical data and provided accurate 15-day-ahead estimates of infections, hospitalisations, ICU admissions and deaths during the whole of 2020. The predictions for 2021 were obtained by assuming the same epidemiological conditions as in the last quarter of 2020 [15,16,17].
2.4 Underlying Mathematical Model and Predictions
The equations for the model describing the COVID-19 transmission dynamics in the Basque Country, as well as the model parameters and initial conditions used for the modelling simulations, are provided in the Electronic Supplementary Material (ESM). The model used is an extension of the Susceptible-Hospitalized-Asymptomatic-Recovered (SHAR) model. To describe the COVID-19 dynamics in the Basque Country, the basic SHAR model was extended to a form called SHARUCD, introducing the classes of ICU admissions, U and deaths, D, and for comparison with the available cumulative empirical data, the cumulative classes of individuals who were infected but asymptomatic/mild cases, were hospitalised, were admitted to an ICU and recovered, CA, CH, CU and CR, respectively, counting all incoming cases in the dynamic compartments and neglecting outflows. A detection ratio ξ for mild/asymptomatic cases was also considered, as a proportion of mild/asymptomatic cases are detected by contact tracing/screening tests, and hence, the number of infections (i.e. cases that have tested positive) is larger than the number of hospitalisations. Disease severity is decided upon infection, a proportion η of cases developing severe infection leading to hospitalisation, and 1 − η experiencing mild/asymptomatic infection. It is assumed that undetected asymptomatic cases transmit the disease more efficiently (φ > 1) than severe cases. Hospitalised individuals can recover, with a recovery rate γ, die, with a mortality rate μ, or be transferred to an ICU, with an admission rate ν. Here, ICU admission is assumed to be a progression of disease severity after hospitalisation. For more information, see the ESM [13, 14, 16, 18].
Once the framework had been calibrated with empirical data and validated with 15-day-ahead predictions for the last quarter of 2020, modelling simulations were obtained for the following months (January to June 2021), assuming similar epidemiological conditions. We note that the SHARUCD model provided the total number of cases of infection, hospitalisations, ICU admissions and deaths in the scenario without vaccination but did not allow subgroup analyses by sex, age, comorbidity or SES.
2.5 Clinical and Socioeconomic Characteristics of Individuals in the Population by COVID-19 Outcome
A nationwide cohort study (2.3 million individuals) was performed using the Basque Health Service database (observed data) from January to June of 2021 to ascertain the determinants of the COVID-19 infection outcomes for each individual in the population (age, sex and Charlson Comorbidity Index [CCI]) in 2021 for the epidemiological scenario with vaccination [6]. Data were gathered on the following variables: sex, SES, diagnosis of COVID-19 infection and infection-related outcomes (uninfected, infected, hospitalised, admitted to an ICU and died). The CCI quantifies the mortality risk associated with 19 weighted comorbidities including congestive heart failure, cerebrovascular disease, chronic lung disease, and diabetes mellitus, and is a significant prognostic factor for infected patients [19,20,21].
To categorise individuals by SES, we used pharmacy co-payment codes, which classify individuals based on their household income [6]. The low-income category (“low SES”) included cases in which the heads of the household were pensioners with a non-contributory pension, disabled individuals or unemployed workers who had exhausted their unemployment benefits. Individuals whose head of household had an income (workers or pensioners with a contributory pension) were classified in the medium-income or high-income category depending on whether the income was < €18,000 (“medium SES”) or ≥ €18,000 (“high SES”). Table SM2 of the ESM provides the characteristics of the Basque population (sex, age group and CCI) in January 2020 according to these SES categories.
For the alternative epidemiological scenario with no vaccination, the individual characteristics of COVID-19-associated outcomes in the first semester of 2021 were assumed to be the same as in the last quarter of 2020. The same variables considered above were analysed, from 1 September to 31 December, 2020 [6]. Instead of building a standard new simulation model [22] by assigning the characteristics separately to infected cases, hospitalised, ICU admissions and deaths, individuals with these outcomes were randomly selected from the entire population in 2021, in a staggered and matched manner to achieve the same distribution as for 2020. First, from the total number of cases predicted by the dynamic model, we disaggregated the number of individuals infected, hospitalised, admitted to an ICU and who died in groups according to the combination of certain variables, namely, sex, age group, CCI and SES, in 2020. To do this, we built a decision tree for each global outcome associated with COVID-19 in which this distribution was taken into account step by step to disaggregate each total outcome into homogeneous subgroups for all variable combinations (sex, age group, CCI, SES). Second, from the population in 2021, we proceeded to randomly select individuals for groups meeting the specified values for each variable. For example, in the first stage, we were able to estimate with the decision tree that the distributions of characteristics of infections in 2020 indicated that 60- to 69-year-old men, with a medium SES and a moderate level of comorbidity represented 2.3% of the 121,743 cases predicted by the SHARUCD model. Therefore, in the second stage, we randomly picked 2800 cases with these characteristics from the 2021 population. In this way, correlations between these variables were ensured, thereby satisfying the assumption that the distribution of characteristics was similar to that of individuals with COVID-19-associated outcomes in 2020.
2.6 Estimating Remaining Life Expectancy Based on Age, Sex and Comorbidity
The remaining comorbidity-adjusted life expectancy was calculated for each individual in the population with one parametric survival function for men and another for women based on year-by-year mortality rates obtained, from the Basque Institute of Statistics (EUSTAT), for 2019–2020 (Table SM1 of the ESM) [23]. The procedure described by Román et al. that determines the lifetime density function using a continuous approach was extended to parameterise survival (Weibull, Gompertz, log-normal, log-logistic) and select the best fit based on R2 values [24]. The life expectancies were validated with the expected survival values estimated by the Spanish Institute of Statistics (INE) in 2019 (see Table SM2 of the ESM). As in cardiovascular risk models, [22] life expectancy was adjusted for each individual’s CCI by including a hazard ratio in the equation according to the individual’s CCI (Table SM3 of the ESM) [21]. Individuals who died had zero life expectancy. To avoid stochastic uncertainty, the same random parameter included in the function was assigned to each individual in both scenarios, with and without vaccination. We applied an annual discount of 3% to the remaining life expectancy [2, 25].
2.7 Utilities and Disutilities Associated with COVID-19 Infection
We estimated the lost QALYs attributable to COVID-19 as the difference between the total remaining comorbidity-adjusted QALYs in the two scenarios. The utility of the remaining life expectancy was obtained using EuroQol EQ-5D-5L scores from the 2012 Spanish Health Survey [26]. Utility was assigned to each individual, according to his/her sex and age (1–29, 30–49, 50–59, 60–69, 70–79 and ≥ 80 years) [22].
As symptoms persist for several months in some infected individuals, we added a disutility to 10% of infected cases and 75% of hospitalised cases to take into account the harmful effect of “long COVID” on the quality of life of patients with symptoms lasting far longer than the initial illness [27]. Specifically, we assumed disutilities of 0.19, 0.30 and 0.50 associated with symptoms lasting for 6 months, 1 year and 2 years for patients infected, hospitalised and admitted to an ICU, respectively [27]. The corresponding number of lost QALYs was calculated by multiplying the duration by the disutility as follows:
2.8 Costs
Average unit costs for healthcare outcomes in 2021 were obtained from the Basque Health Service Accounting System (Table SM4 of the ESM). Infected cases were assumed to result in two visits to a general practitioner (€118). Hospitalisation costs were estimated based on summing the average costs of a ward stay and follow-up by outpatient services (€7919). Admission to an ICU had a higher average cost, including both more outpatient consultations and rehabilitation (€36,345). We did not take into account the costs of contact tracing or polymerase chain reaction tests for cases and contacts.
Both the official prices and actual prices paid were used to estimate the cost of the vaccination programme. The European Commission bought the vaccines at below the official market price. Specifically, the prices paid for vaccines (Moderna, €18; Pfizer-BioNTech, €12; Oxford-AstraZeneca, €1.78; and Johnson & Johnson’s Janssen, €8.50) as posted on Twitter by Belgium’s Secretary of State for the Budget (https://www.theguardian.com/world/2020/dec/18/belgian-minister-accidentally-tweets-eus-covid-vaccine-price-list) were substantially lower than the official prices (Moderna, €31; Pfizer-BioNTech, €17; Oxford-AstraZeneca, €3; and Johnson & Johnson’s Janssen, €8).
3 Results
Table 1 presents the differences in total outcomes and costs for the two scenarios considered, namely, with and without vaccination. The savings due to vaccines reached an estimated €26 million. The good fit between the characteristics of the populations infected in 2020 and simulated for 2021 can be seen in Fig. SM1 of the ESM. Table 2 describes the characteristics of the infected and uninfected individuals during the last quarter of 2020 and the first half of 2021.
The characteristics of the individuals with the four COVID-19-related outcomes in the two scenarios are compared in Tables 3 and 4 and Tables SM6 and SM7 of the ESM. The largest difference was observed in the distribution of hospitalisations by CCI, which evidenced that vaccination avoided a considerable percentage of severe cases in individuals with comorbidities. On the contrary, the percentages of avoided infections were similar in all categories.
After the assignment of characteristics for each avoided outcome, we calculated the life expectancy (Table SM8 of the ESM) and quality-adjusted life expectancy of the population in each scenario, which shows that the gain in years of life was greater in the younger groups. The average life expectancy for the population was 34.812 years with vaccination and 34.791 years without vaccination. Adjusting for quality of life and the discount yielded QALY values of 15.855 and 15.849, respectively. The average individual gains for the total population were 0.021 years and 0.006 discounted QALYs.
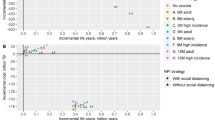
Vaccines costed €32.44 million based on the official prices and €22.17 million considering the prices paid. The total costs saved were estimated at €26.44 million resulting from the multiplication of unit costs by avoided outcomes (Table 1). The ICER of COVID-19 vaccines was €707/QALY with official vaccine prices and was dominant with real prices (Table 5). When disaggregating the cost-effectiveness analysis by SES to estimate the distributional ICER, the result did not render clear differences between income categories. By contrast, considering comorbidities, there were marked differences between groups with low (CCI 0, CCI 1–2) and high levels of comorbidity (CCI 3–4, CCI > 4), as shown in Table 5 and Table SM9 of the ESM with confidence intervals for incremental cost and utility. The ICER was always dominant for the latter, with both official and real prices, but it was never dominant for the healthiest individuals (CCI 0). Last, there were differences by sex, the ICER being dominant for men and positive for women. The distributional cost-effectiveness plane (incremental cost on the vertical axis and incremental effectiveness on the horizontal axis) with confidence intervals for the ICER, presented in Fig. 1, indicates the same results.
Cost-effectiveness plane (incremental cost on the vertical axis and incremental effectiveness on the horizontal axis) with confidence intervals for the analysis disaggregated by sex (a), socioeconomic status [SES] (b) and level of comorbidity (c). CCI Charlson Comorbidity Index, QALY quality-adjusted life-year
4 Discussion
This study presents a cost-utility analysis of COVID-19 vaccination based on the actual infection outcomes in a nationwide cohort of 2.3 million individuals disaggregated by comorbidity and SES. Although recently published studies have explored the health and economic impacts of COVID-19 vaccination by considering different vaccination strategies and future scenarios, we are unaware of any studies that have measured the actual health and economic value of vaccination programmes in a real-world population [11, 23, 28]. Our innovative approach combined the results of a dynamic model to estimate the scenario with no vaccination, with the analysis of the whole population to identify CCI and SES, and techniques from parametric survival to achieve individual-level results for health and costs associated with COVID-19 infection. It is noteworthy that the synergies between modelling and real-world data to achieve full economic evaluations are reinforced by the use of common data sources [6, 14, 16, 18]. Our results answer an important question about the efficiency of the vaccination programme for different groups as a function of clinical and social determinants, confirming that the priority given to people with comorbidities was well justified. More importantly, our findings have shown some indicators of inequity during the vaccination rollout in the Basque Country, probably owing to a lower adherence to vaccination. The higher percentage of avoided deaths in the low SES could be understood as evidence of equity. However, we found that the low SES group achieved less gain than the other groups when we calculated the benefit of vaccines as the mean remaining comorbidity-adjusted life expectancy of the population according to age, SES and CCI in the scenarios with and without vaccination. Our interpretation is that the differences in the percentage of avoided deaths are biased by the higher comorbidity of the low SES group and the reality is that people with comorbidity in the higher SES groups benefited more from the vaccination.
The label of dominance in an economic evaluation means that the treatment under evaluation provides more QALYs and saves healthcare costs and, therefore, it should be adopted [2]. In the Basque Country, COVID-19 vaccination was dominant from the perspective of the healthcare payer when real prices were used, consistent with the efficiency of vaccines shown by studies analysing potential scenarios [12, 25]. To avoid misunderstandings, we underline that our modelling results are not based on the analysis of hypothetical scenarios of selective delivery of vaccines to each subgroup but on a single incremental cost-effectiveness analysis of a “real” vaccination strategy compared to a counterfactual strategy of non-vaccination. We have only carried out one application of the SHARUCD model and its results have been projected in the complete OAS database. The procedure for randomly assigning the results associated with COVID that took into account the probability of the different categories of sociodemographic and comorbidity variables made it possible to obtain individual data on the cost and effectiveness of each subgroup in the scenario without vaccines based on the combination of the dynamic model and the OAS database. The same information about the vaccination alternative was already available in the OAS database.
Our findings on the ICER did not render clear differences between low-SES, medium-SES, and high-SES groups. However, using the CCI to classify population comorbidity prior to COVID-19 infection showed that the programme was more efficient in the highest comorbidity groups (CCI > 4). These results justify the vaccine administration priority criteria applied by the Basque Public Health System, in agreement with its Beveridge model, implementing a centralised rollout of vaccines and giving priority to elderly and high-risk individuals. By the end of June 2021, the vaccination programme in the Basque Country had administered vaccines to 95% of ≥ 70-year-olds, the age group with the highest rates of comorbidity. By contrast, younger people had fewer comorbidities and their full vaccination percentages were much lower at this stage. Despite 20% of the population in the Basque Country having double healthcare coverage (public and private), access to the vaccination programme was only possible through the public system, ensuring equity in terms of SES in vaccine distribution. The lack of a clear social gradient in our results may be surprising as the literature is abundant on an increased risk of infection and an increased risk of death in deprived areas [29]. However, in 2021, the greatest risk of contagion in the Basque population occurred in the groups between 15 and 50 years of age because of their greater socialisation, to a large extent in leisure venues, which correlates with a greater availability of income [6]. In addition, the prioritisation of groups with higher comorbidity in the implementation of vaccination boosted vaccination in lower-income groups as they had higher comorbidity. The possible barriers to accessibility of lower income groups were solved by a vaccination rollout carried out in a centralised and proactive manner, reinforcing the primarycCare network that covers 100% of the population.
The SHARUCD model developed to guide decision making by public health managers during the first year of the pandemic was used to estimate the number of cases expected in a scenario in which vaccination had not been implemented. Giving predictions validated with the official data from February to December 2020, the simulations were obtained without adding any control function for January to June 2021. For interpreting the model, we assumed that, in the absence of a vaccination rollout, the behaviour of the pandemic in the first semester of 2021 would have followed the same trend as observed in the last months of 2020, i.e. a situation before COVID-19 antibodies started to decline, reflecting waning immunity, and before the Delta variant became dominant. This assumption was justified by empirical data showing similar patterns in the epidemic waves reported from the last months of 2020 until the end of June of 2021, with incidence rates and reproduction numbers in similar ranges to those associated with the same COVID-19 Alpha variant [15]. A comparison of model simulations with official data for the first half of 2021 indicated that the initial phase of the vaccination rollout was responsible for reducing infections, hospitalisations, ICU admissions and deaths associated with COVID-19 by 25.5%, 16.0%, 19.6% and 18.4%, respectively [30].
The SES, CCI, sex and age of the population had only a small influence on the risk of infection, the percentage of avoided infections not varying substantially as a function of these variables. On the contrary, differences did appear among patients with more severe disease; specifically, we found: (1) proportionally fewer hospitalisations in ≥70-year-olds, patients with CCI scores of 3–4, and those with high SES, and (2) proportionally fewer ICU admissions in men, patients with high SES, and 50- to 69-year-olds. Further, proportionally more deaths were avoided, indicating a greater benefit from the vaccination programme, in some groups, namely, patients with high levels of comorbidity (CCI score > 2) and ≥ 70-year-olds. As men had more comorbidities than women, they benefited more from vaccination, their ICER being dominant while that of women was positive.
Last, but not least, we underline the importance of knowing the real costs of drugs to carry out realistic economic evaluations, as highlighted by the results described here. In the case of the real costs of the COVID-19 vaccines used in the European Union, this information was released by an “accidental data leak” revealing the savings in the prices obtained by centralised purchasing. Notably, the analysis with the real vaccine prices rendered, from the perspective of the healthcare payer, a dominant ICER.
Our work is not exempt from limitations. The main limitations relate to the lack of a comprehensive approach for estimating the cost of each scenario by incorporating indirect costs and the macroeconomic perspective considering the impact of the pandemic in terms of drops in gross domestic product [4]. Moreover, while the vaccination rollout was still ongoing at the 6-month time horizon, our approach was not able to include all the real benefits from vaccines remaining effective for a longer period. Another limitation to mention is the lack of adjustment of comorbidity or SES on quality of life. This means that the QALY gains and cost effectiveness of vaccinating comorbid or low SES individuals may have been overestimated. We can justify this omission on the grounds of seeking to avoid the potential risk of indirect disability or social discrimination. Additionally, we have to mention that the best calibration would have been carried out by introducing vaccines to the model, but this adaptation was not available and we proceeded by running the model according to the calibration to the last quarter of 2020.
5 Conclusions
Finally, we conclude that the analysis by comorbidity showed that vaccines were considerably more cost effective in individuals with pre-existing health conditions. However, this benefit was lower in the low SES group. The economic evaluation of the vaccination programme justified the policy of prioritising high-risk patients. The initial phase of COVID-19 vaccination was dominant from the perspective of the healthcare payer.
References
Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–29.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2005.
Elvidge J, Summerfield A, Nicholls D, Dawoud D. Diagnostics and treatments of COVID-19: a living systematic review of economic evaluations. Value Health. 2022;25:773–84.
Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324:1495–6.
Anderson RM, May RM. Directly transmitted infections diseases: control by vaccination. Science. 1982;215:1053–60.
Ibarrondo O, Aguiar M, Stollenwerk N, Blasco-Aguado R, Larrañaga I, Bidaurrazaga J, et al. Changes in social and clinical determinants of COVID-19 outcomes achieved by the vaccination program: a nationwide cohort study. Int J Environ Res Public Health. 2022;19:12746.
Khanijahani A, Iezadi S, Gholipour K, Azami-Aghdash S, Naghibi D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. 2021;20:248.
Woodward M, Peters SAE, Harris K. Social deprivation as a risk factor for COVID-19 mortality among women and men in the UK Biobank: nature of risk and context suggests that social interventions are essential to mitigate the effects of future pandemics. J Epidemiol Community Health. 2021;75:1050–5.
Marí-Dell’Olmo M, Gotsens M, Pasarín MI, Rodríguez-Sanz M, Artazcoz L, Garcia de Olalla P, et al. Socioeconomic inequalities in COVID-19 in a European urban area: two waves, two patterns. Int J Environ Res Public Health. 2021;18:1256.
Meunier A, Longworth L, Kowal S, Ramagopalan S, Love-Koh J, Griffin S. Distributional cost-effectiveness analysis of health technologies: data requirements and challenges. Value Health. 2023;26:60–3.
Sandmann FG, Davies NG, Vassall A, Edmunds WJ, Jit M; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: a transmission model-based future scenario analysis and economic evaluation. Lancet Infect Dis. 2021;21:962–74.
Hagens A, İnkaya AÇ, Yildirak K, Sancar M, van der Schans J, Acar Sancar A, et al. COVID-19 vaccination scenarios: a cost-effectiveness analysis for Turkey. Vaccines. 2021;9:399.
Aguiar M, Anam V, Cusimano N, Knopoff D, Stollenwerk N. Understanding COVID-19 epidemics: a multi-scale modeling approach. In: Bellomo N, Chaplain MAJ, editors. Predicting pandemics in a globally connected world. Vol 1. Cham: Springer; 2022: p. 11–42. https://doi.org/10.1007/978-3-030-96562-4_2.
Aguiar M, Van-Dierdonck JB, Mar J, Cusimano N, Knopoff D, Anam V, et al. Critical fluctuations in epidemic models explain COVID-19 post-lockdown dynamics. Sci Rep. 2021;11:13839.
Galán JC, Cantón R. New variants in SARS-CoV-2: what are we learning from the omicron variant? Arch Bronconeumol. 2022;58:3–5.
Aguiar M, Ortuondo EM, Bidaurrazaga Van-Dierdonck J, Mar J, Stollenwerk N. Modelling COVID 19 in the Basque Country from introduction to control measure response. Sci Rep. 2020;10:17306.
Aguiar M, Van-Dierdonck JB, Mar J, Stollenwerk N. The role of mild and asymptomatic infections on COVID-19 vaccines performance: a modeling study. J Adv Res. 2022;39:157–66.
Srivasrav AK, Stollenwerk N, Bidaurrazaga Van-Dierdonck J, Mar J, Ibarrondo O, Aguiar M. Modeling the initial phase of COVID-19 epidemic: the role of age and disease severity in the Basque Country, Spain. PLoS ONE. 2022;17: e0267772.
Tuty Kuswardhani RA, Henrina J, Pranata R, Anthonius Lim M, Lawrensia S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:2103–9.
Cho SI, Yoon S, Lee H-J. Impact of comorbidity burden on mortality in patients with COVID-19 using the Korean health insurance database. Sci Rep. 2021;11:6375.
Bannay A, Chaignot C, Blotière P-O, Basson M, Weill A, Ricordeau P, et al. The best use of the Charlson Comorbidity Index with electronic health care database to predict mortality. Med Care. 2016;54:188–94.
Arrospide A, Ibarrondo O, Castilla I, Larrañaga I, Mar J. Development and validation of a discrete event simulation model to evaluate the cardiovascular impact of population policies for obesity. Med Decis Mak. 2022;42:241–54.
Liu Y, Sandmann FG, Barnard RC, Pearson CAB, Pastore R, Pebody R, et al. Optimising health and economic impacts of COVID-19 vaccine prioritisation strategies in the WHO European Region: a mathematical modelling study. Lancet Reg Health Eur. 2022;12: 100267.
Román R, Comas M, Hoffmeister L, Castells X. Determining the lifetime density function using a continuous approach. J Epidemiol Community Health. 2007;61:923–5.
Kohli M, Maschio M, Becker D, Weinstein MC. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: Use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39:1157–64.
Arrospide A, Machón M, Ramos-Goñi JM, Ibarrondo O, Mar J. Inequalities in health-related quality of life according to age, gender, educational level, social class, body mass index and chronic diseases using the Spanish value set for Euroquol 5D–5L questionnaire. Health Qual Life Outcomes. 2019;17:69.
Chevinsky JR, Tao G, Lavery AM, Kukielka EA, Click ES, Malec D, et al. Late conditions diagnosed 1–4 months following an initial COVID-19 encounter: a matched cohort study using inpatient and outpatient administrative data, United States, March 1-June 30, 2020. Clin Infect Dis. 2021;73(Suppl. 1):S5-16.
Jahn B, Sroczynski G, Bicher M, Rippinger C, Mühlberger N, Santamaria J, et al. Targeted COVID-19 Vaccination (TAV-COVID) considering limited vaccination capacities: an agent-based modeling evaluation. Vaccines. 2021;9:434.
Politi J, Martín-Sánchez M, Mercuriali L, Borras-Bermejo B, Lopez-Contreras J, Vilella A, et al. Epidemiological characteristics and outcomes of COVID-19 cases: mortality inequalities by socio-economic status, Barcelona, Spain, 24 February to 4 May 2020. Euro Surveill. 2021;26:2001138.
Marshall DA, Burgos-Liz L, IJzerman MJ, Crown W, Padula WV, Wong PK, et al. Selecting a dynamic simulation modeling method for health care delivery research-part 2: report of the ISPOR Dynamic Simulation Modeling Emerging Good Practices Task Force. Value Health. 2015;18:147–60.
Acknowledgements
We acknowledge the help of Ideas Need Communicating Language Services in improving the use of English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by the Basque Foundation for Health Innovation and Research (Grant number BIO21/COV/001), the Basque Government, through the “Mathematical Modeling Applied to Health” Project and the BERC 2022-2025 programme, and the Spanish Ministry of Science, Innovation, and Universities, through the Severo Ochoa accreditation (SEV-2017-0718) of the Basque Center for Applied Mathematics. The funding sources had no involvement in the study design in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article.
Conflicts of interest/competing interests
Javier Mar, Oliver Ibarrondo, Carlo Delfin S. Estadilla, Nico Stollenwerk, Fernando Antoñanzas, Rubén Blasco-Aguado, Igor Larrañaga, Joseba Bidaurrazaga and Maíra Aguiar have no biomedical financial interests or potential conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study protocol (ref: EOM2021029) was approved by the Ethics and Clinical Research Committee of the Basque Country on 18 May, 2021. This study was performed in line with the principles of the Declaration of Helsinki and received approval from the Ethics and Clinical Research Committee of Euskadi (study code EOM2021029), which waived the informed consent as all data were anonymised.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data were provided by the Basque Health Service. Our data sharing agreement clearly stipulates that they cannot be shared with any third party.
Code availability
Not applicable.
Authors’ contributions
JM and MA conceived and designed the research. JB provided the epidemiological data and analysed the COVID-19 outcomes. OI, FA and IL obtained the data, performed the simulated and observed analyses, interpreted the data and drafted the results section. MA, RB, CDSE and NS developed and analysed the dynamic model, and wrote the corresponding methods section. JM, MA, FA and OI drafted the manuscript. All authors revised the manuscript for important intellectual content and approved the final version. Further, they all had full access to all the data used in the study and accepted responsibility to submit for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mar, J., Ibarrondo, O., Estadilla, C.D.S. et al. Cost-Effectiveness Analysis of Vaccines for COVID-19 According to Sex, Comorbidity and Socioeconomic Status: A Population Study. PharmacoEconomics 42, 219–229 (2024). https://doi.org/10.1007/s40273-023-01326-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01326-y