Abstract
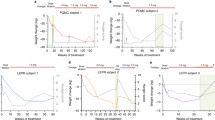
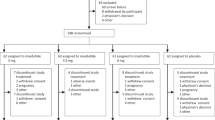
Subcutaneous setmelanotide (IMCIVREE®), a melanocortin-4 receptor agonist, meets a previously unmet need and thus represents an important advancement in the management of patients with pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1) or leptin receptor (LEPR) deficiency obesity. Setmelanotide is the first approved treatment in patients aged ≥ 6 years with these conditions. In two single-arm, open-label, multicentre, phase III trials, subcutaneous setmelanotide was effective in reducing body weight by at least 10% from baseline after approximately 1 year of treatment at individualised therapeutic doses in patients aged ≥ 6 years with POMC, PCSK1 or LEPR deficiency obesity. Other outcomes relating to weight loss and self-reported hunger scores were also improved with setmelanotide therapy. Setmelanotide was generally well tolerated in clinical trials.
Plain Language Summary
The causes of obesity are multifactorial, one of which is genetic mutations leading to deficiencies in key ligands and receptors involved in the leptin-melanocortin pathway in the brain that regulates hunger and body weight. While lifestyle interventions and bariatric surgery are available, there is a lack of pharmacological treatment options for obesity arising from genetic factors. Subcutaneous setmelanotide (IMCIVREE®) is the first treatment to be approved in the EU and the USA for patients aged ≥ 6 years with obesity due to pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1) or leptin receptor (LEPR) deficiency. It works by activating the melanocortin-4 receptor, a crucial protein receptor in the leptin-melanocortin pathway, facilitating weight loss through hunger reduction and increased energy expenditure. In phase III trials, approximately one year of treatment with subcutaneous setmelanotide was associated with at least 10% weight reduction in a significant number of patients with POMC, PCSK1 or LEPR deficiency obesity. Improvements in other outcomes related to weight loss and self-reported hunger scores were also evident. Setmelanotide was generally well tolerated. Setmelanotide represents an important advancement in the management of patients with POMC, PCSK1 or LEPR deficiency obesity.



Similar content being viewed by others
References
Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol. 2021;12:706978.
Yeo GSH, Chao DHM, Siegert AM, et al. The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol Metab. 2021;48:101206.
Poitou C, Puder L, Dubern B, et al. Long-term outcomes of bariatric surgery in patients with bi-allelic mutations in the POMC, LEPR, and MC4R genes. Surg Obes Relat Dis. 2021;17(8):1449–56.
Hainer V, Aldhoon Hainerova I, Kunesova M, et al. Melanocortin pathways: suppressed and stimulated melanocortin-4 receptor (MC4R). Physiol Res. 2020;69(Suppl 2):S245–54.
Clément K, van den Akker E, Argente J, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endo. 2020;8(12):960–70.
Huvenne H, Dubern B, Clément K, et al. Rare genetic forms of obesity; clinical approach and current treatments in 2016. Obes Facts. 2016;9(3):158–73.
Durrer Schutz D, Busetto L, Dicker D, et al. European practical and patient-centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12(1):40–66.
Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–62.
Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity—assessment, treatment, and prevention: and Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–57.
National Institute for Health and Care Excellence. Obesity: identification, assessment and management. 2021. https://www.nice.org.uk/guidance/cg189. Accessed 7 June 2022.
Yanovski SZ, Yanovski JA. Progress in pharmacotherapy for obesity. JAMA. 2021;326(2):129–30.
Reinehr T. Treatment of children with obesity [abstract no. TS08-02]. Obes Rev. 2020;21(Suppl 1):11–2.
Clément K, Biebermann H, Farooqi IS, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med. 2018;24(5):551–5.
Cignarella A, Busetto L, Vettor R. Pharmacotherapy of obesity: an update. Pharmacol Res. 2021;169: 105649.
Rhythm Pharmaceuticals. IMCIVREE (setmelanotide): EU summary of product characteristics. 2021. https://www.ema.europa.eu. Accessed 7 June 2022.
Rhythm Pharmaceuticals. IMCIVREE (setmelanotide) injection, for subcutaneous use: US prescribing information. 2020. https://www.accessdata.fda.gov. Accessed 7 June 2022.
Markham A. Setmelanotide: first approval. Drugs. 2021;81(3):397–403.
Kühnen P, Clément K, Wiegand S, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. NEJM. 2016;375(3):240–6.
Thompson Z, Shah BP, Low MJ. Chronic treatment of juvenile hypothalamic pomc-deficient mice with RM-493 prevents the development of obesity [abstract no. SAT-299]. J Endocr Soc. 2020;4(Suppl 1):A166–7.
Chen KY, Muniyappa R, Abel BS, et al. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. J Clin Endocr Metab. 2015;100(4):1639–45.
Kievit P, Halem H, Marks DL, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62(2):490–7.
Clément K, Argente J, Bahm A, et al. Long-term weight and hunger reduction with setmelanotide in individuals with POMC deficiency obesity [abstract no. 265]. Obesity (Silver Spring). 2020;28(Suppl 2):120.
Kühnen P, Wabitsch M, von Schnurbein J, et al. Quality of life outcomes in two phase 3 trials of setmelanotide in patients with obesity due to LEPR or POMC deficiency. Orphanet J Rare Dis. 2022;17(1):38.
Wabitsch M, Fehnel S, Mallya UG, et al. Understanding the patient experience of hunger and improved quality of life with setmelanotide treatment in POMC and LEPR deficiencies. Adv Ther. 2022;39(4):1772–83.
European Medicines Agency. IMCIVREE (setmelanotide): EMA public assessment report. 2021. https://www.ema.europa.eu. Accessed 7 June 2022.
Clément K, van den Akker ELT, Gordon G. Timing of onset of adverse events with setmelanotide, an MC4R agonist, in patients with severe obesity due to LEPR or POMC deficiency [abstract no. P02–51 plus poster]. J Endocr Soc. 2021;5(Suppl 1):A30-1.
Sharma S, Garfield AS, Shah B, et al. Current mechanistic and pharmacodynamic understanding of melanocortin-4 receptor activation. Molecules. 2019;24(10):1892.
Acknowledgements
The manuscript was reviewed by: L. Busetto, Department of Medicine, University of Padova, Padova, Italy; F. Cadegiani, Universidade Federal de São Paulo, São Paulo, Brazil. During the peer review process, Rhythm Pharma Inc., the marketing authorization holder of setmelanotide, was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
C. Kang is a salaried employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, C. Setmelanotide in obesity: a profile of its use. Drugs Ther Perspect 38, 308–315 (2022). https://doi.org/10.1007/s40267-022-00929-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00929-3