Abstract
Background
Elderly people who reside in long-term care facilities form a frail and vulnerable population, with multiple pathologies and high percentages of cognitive and functional disability.
Objectives
The aims of this study were to assess the safety of vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in frail nursing home residents and to evaluate its effectiveness 6 months after full vaccination.
Design
This was an ambispective observational study.
Setting
Residents of a long-term care facility in Madrid, Spain.
Participants
One hundred and thirty-seven nursing home residents (81.8% female, mean age 87.77 ± 8.31 years) with high comorbidity (61.3% Charlson Index ≥ 3) and frailty (75% Clinical Frail Scale ≥ 7) who received the BNT162B2 mRNA vaccine.
Measurements
Safety data were collected to evaluate the type of adverse drug reactions and their duration, severity, and causality. Immunogenicity was tested 6 months after the primary vaccination and effectiveness was evaluated by the incidence of SARS-CoV-2 infection, the number of hospital admissions, and mortality due to coronavirus disease 2019 (COVID-19).
Results
Safety: Of the residents, 21.9% had some adverse reaction and 5.8% had a severe or more serious adverse reaction. The most frequent adverse reactions were fatigue (13.1%), pyrexia (12.4%), and headache (7.3%). No association was observed between frailty (including a need for palliative care) and clinical, functional or cognitive status of the participants and the occurrence of adverse events. Immunogenicity and Effectiveness: After 6 months of vaccination, only one case of SARS-CoV-2 infection was confirmed in the vaccinated residents. Most of the nursing home residents presented positive serology (95.2%). Loss of immunogenicity was associated with older age (95.12 ± 3.97 vs. 87.24 ± 8.25 years; p = 0.03) and no previous COVID-19 infection (16.6% vs. 70%; p < 0.001). Binary logistic regression models did not reveal this association.
Conclusion
The BNT162B2 vaccine is well tolerated and effective in nursing home residents, independently of their clinical, functional, cognitive, or frailty characteristics. For the most part, immunogenicity has been maintained over time, regardless of comorbidity, functional status or frailty.
Similar content being viewed by others
Vaccination in elderly subjects residing in nursing homes does not carry an increased risk of adverse effects. |
With the data available to date, there is no reason to contraindicate vaccination in the older age groups, not even in frail nursing home residents. |
1 Introduction
Elderly people have been most affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (coronavirus disease 2019 [COVID-19]), especially those who reside in long-term care facilities (LTCFs). High mortality in older adults, especially in the most frail older adults, has motivated the Spanish health authorities to include residents of LTCFs in the highest risk groups and they were subsequently the first to be vaccinated.
In April 2021, the immunization of this priority group was considered almost complete, with a total of 300,133 residents of LTCFs having received the full regimen [1]. The vaccines used in this group were the messenger RNA (mRNA) vaccine BNT162b2 (Pfizer/BioNTech) and the mRNA-1273 vaccine (Moderna). Authorized COVID-19 vaccines have demonstrated efficacy and safety in clinical trials; however, COVID-19 vaccine trials have generally excluded the frail elderly and residents of LTCFs [2, 3]. In the BNT162b2 trial, only 4.4% of the included individuals were aged 75 years or older [4].
By October 2021, more than 70 million doses of the COVID-19 vaccine had been administered in Spain (70% of which were for BNT162b2), vaccinating 37.2 million people, 23.2% of whom were over 65 years of age (8.7 million) [5]. By that date, a total of 46,573 adverse events following vaccination had been reported to the Spanish Pharmacovigilance System for Medicinal Products for Human Use (SEFV-H), of which 9430 were considered serious adverse events. Of the 46,573 events reported, 4574 occurred in people over 65 years of age. However, the reported adverse events cannot be considered as adverse reactions due to the vaccine until the causal relationship with the administration of the vaccine is confirmed.
The most commonly reported adverse events following administration of the mRNA BNT162b2 vaccine were pyrexia (35%), headache (25%), myalgia (18%), pain at the vaccination site (12%), malaise (12%), fatigue (8%), nausea (7%), chills (7%), lymphadenopathy (7%), and asthenia (7%) [5].
Elderly people living in nursing homes form a frail population, with multiple pathologies and high percentages of cognitive and functional disability. Only a few studies on COVID-19 vaccination and the incidence of adverse reactions according to clinical, functional, or cognitive status have been identified [3]. In this respect, the Norwegian Medicines Agency investigated 13 deaths in very frail nursing home residents and concluded that common adverse reactions to mRNA vaccines, such as fever, nausea and diarrhea, may have contributed to these fatal outcomes without being able to specify causality [6].
The aim of this study was to assess the safety of vaccination against SARS CoV-2 infection using real-life data in nursing home residents with high comorbidity, frailty, and cognitive and/or functional disability, and to evaluate its effectiveness and immunogenicity 6 months after full vaccination.
2 Methods
An ambispective observational study was conducted between January and October 2021 among residents of a public LTCF of the AMAS (Agencia Madrileña de Atención Social) in Madrid, Spain. Sociodemographic, clinical, functional, and cognitive variables were collected prospectively between administration of the first and second doses. Safety data were collected retrospectively from the first dose, and prospectively after the administration of the second dose. Effectiveness was evaluated prospectively from full vaccination and up to 6 months follow-up. Complete vaccination or primary vaccination was considered in this study for those vaccinated with two doses of the mRNA vaccine.
Inclusion criteria were living in a nursing home during the vaccination period and consent to participate in the study. Excluded from the safety study were those who had not received the vaccine, for whatever reason.
The presence of comorbidities was evaluated using the Charlson Index and subsequently categorized into two grades: low (0–2 points) or high (≥ 3 points) comorbidity. Functional status was measured using the Barthel Index and was subsequently categorized according to different grades, such as independent (IB = 100 points), mild dependence (60–99), moderate dependence (40–59), severe dependence (20–39), and total dependence (0–19). Frailty was evaluated using two scales: (1) the Clinical Frailty Scale (Rockwood CFS) [7], which was categorized into non-frail (CFS 1–4), mild–moderate frailty (CFS 5–6), or severe frailty (CFS 7–9); and (2) the Frailty Index-VIG (FI-VIG) [8], categorizing participants as non-frail (0–0.2 points), mild frailty (> 0.2 to 0.4), moderate frailty (0.4–0.6), and advanced frailty with a need for palliative care (> 0.6). Nutritional status was evaluated using the Mini Nutritional Assessment Short (MNA-Short), resulting in the subsequent categorization of three nutritional statuses: malnutrition (0–7 points), risk of malnutrition (8–11 points), and normal nutritional status (12–14 points). The presence of dementia was measured according to the Reisberg Global Deterioration Scale (GDS), with grades of mild (GDS 4), moderate (GDS 5), and severe (GDS 6-7) dementia. Evidence of SARS-CoV-2 infection previous to vaccination was collected, together with date and diagnostic test used (reverse transcription polymerase chain reaction [RT-PCR] tests, serological test, or rapid antigen test) [9]
Safety data were collected from the 137 subjects who received the vaccine, during the first and second administration. To evaluate safety, data on the type of adverse drug reactions (ADRs) and their duration and severity were collected. The grading scales used in this study to assess safety were derived from the FDA Center for Biologics Evaluation and Research (CBER) guidelines on toxicity grading scales for healthy adult volunteers enrolled in preventive vaccine clinical trials, and were classified as mild (grade 1), moderate (grade 2), severe (grade 3), or life-threatening (grade 4) [10].
The causality of the adverse reactions produced was assessed and classified using the algorithm used by the Spanish Pharmacovigilance System [11], which evaluates the following aspects: the temporal sequence, the previous description of the adverse effect in the data sheet, the evolution of the adverse effect after administration of the vaccine, the effect of re-administration, the existence of alternative causes, and the existence of contributing factors favoring the causal relationship. According to the score obtained, causality was classified into five categories: unrelated (< 0 points), conditional (1–3 points), possible (4–5 points), probable (6–7 points), or definite (10 points).
Effectiveness was evaluated by the incidence of SARS-CoV-2 infection, number of hospital admissions, and mortality due to COVID-19 6 months after the second dose. Additionally, immunogenicity was tested 6 months after the primary vaccination. Specific antibodies in blood were measured using a qualitative chemiluminescent immunoassay (CLIA) that detects immunoglobulin (Ig) G antibodies to the S1 subunit of the spike protein (Ortho Clinical Diagnostics). Test results are based on the ratio of the serum reading (S) to the cut-off reading (C), with S/C values ≥ 1 being considered positive. S/C values reflect relative values of anti-SARS-CoV-2 IgG levels.
Data collection was performed by the Centre’s healthcare staff (geriatrician, nurse, and pharmacist) and data analysis was performed by staff of the referral hospital (geriatrician and hospital pharmacist). Continuous data were presented as mean ± standard deviation (SD) and qualitative data were presented as frequency (%). Differences were analyzed using the Chi-square and Fisher’s test, Student’s t test, Mann–Whitney U test, or analysis of variance as indicated. Binary logistic regression models were constructed to analyze the adjusted variables associated with the presence of ADR vaccination and antibody up to 6 months from vaccination in this population. The statistical analysis software SPSS® version 26 (IBM Corporation, Armonk, NY, USA) was used for analysis of the variables.
This study was approved by the Local Research Ethics Committee, registration code PI-4616. All participants signed the informed consent form.
3 Results
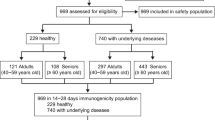
Among a total of 144 residents, 2 were excluded because they refused to participate in the study, resulting in a study sample consisting of 142 nursing home residents, of whom 137 (96.48%) received the first two doses. Five individuals refused vaccination (3.52%), three due to family refusal and two due to medical indication (risk of anaphylaxis) (Fig. 1).
The mRNA vaccine BNT162b2 (Pfizer/BioNTech) was administered, with the first dose being administered between 12 January and 23 April 2021, and the second between 1 February and 13 April 2021, with an interval of 20.3 ± 3.9 days between the two doses.
3.1 Safety
The sociodemographic, clinical, functional, and cognitive characteristics of the participants who received the vaccine are displayed in Table 1. The median age was 87.77 years (range 60–103). Most participants in the safety study were female (81.8%), with a high presence of comorbidities (61.3%, Charlson Index ≥ 3 points) and severe frailty (75% CFS > 7–9). Among the 137 study participants who received the vaccine, 70.8% had passed COVID-19 in the past and 62.0% had a positive prevaccination serology.
Thirty residents (21.9%) reported an adverse event following vaccination, of whom 8 (5.8%) reported a severe or greater adverse event. Only one resident had a grade 4 adverse event (potentially life-threatening). Overall, the most frequently reported adverse effects were fatigue (13.1%), fever (12.4%), and headache (7.3%). After administration of the first dose, headache and fatigue were the most frequent adverse effects, whereas fever and fatigue were the most frequent adverse effects after the second dose (Table 2). There was one case of syncope and one case of myoclonus after the first dose, both of which were severe but resolved in < 24 h.
A case of leukocytoclastic vasculitis occurred in a patient with severe dementia, starting 5 days after receiving the second dose and requiring hospitalization. It was treated with low-dose corticosteroids and after 7 days of hospitalization, the resident returned to his nursing home without sequelae under the care of two geriatricians (hospital and nursing home). The causality algorithm determined that a causal relationship of the vasculitis with the vaccine was ‘possible’.
The factors associated with ADRs have been evaluated after both the first and second doses, and no statistically significant associations were found. A new global analysis of all the ADRs that appeared (first and second doses) was performed, as shown in Table 1. A total of 81 ADRs were reported, most of which were mild or moderate (77.8%), 13.6% were severe, and 1.2% were life-threatening. There was a higher percentage of moderate or severe adverse reactions after administration of the first dose (Fig. 2a, b). A possible causal relationship was established for 64.3% of the reactions after the first dose and a probable causal relationship was established for 73.6% of the reactions after the second dose (Fig. 2c, d). In a bivariate analysis, no association was observed between frailty (including a need for palliative care); clinical, functional, or cognitive status of the participants; and the occurrence of adverse events (Table 1). It was observed that subjects with COVID-19-positive serology presented more frequently with severe ADRs (9.4% vs. 0%; p = 0.024). In a multivariate analysis using a linear regression model, no particular variable was associated with the presence of severe ADRs.
3.2 Immunogenicity and Effectiveness
Until October 2021, only one case of SARS-CoV-2 infection was confirmed by RT-PCR in the 137 vaccinated residents, with a self-limited fever and diarrhea that required social-health follow-up due to COVID-19. After 6 months of vaccination (233 ± 22.4 days), 11 deaths from other causes were reported, unrelated to COVID-19. After this follow-up, most of the nursing home residents presented positive serology, and only six individuals (4.76%) showed negative serology for SARS-CoV-2 (Table 3). Differences were observed regarding the age of participants with negative serology after vaccination versus those with positive serology; loss of immunogenicity was associated with older age (95.18 ± 3.97 vs. 87.24 ± 8.25 years; p = 0.03). Differences regarding immunogenicity were also observed, with higher levels found in those who previously had COVID-19 (70% vs. 16.6%; p = 0.009) or those with positive serology prior to vaccination (63.3% vs. 0%; p = 0.02) (Table 3). In a multivariate analysis using a linear regression model, no particular variable was associated with the presence of serology after 6 months. Co-linearity was not present among the variables included in the model.
4 Discussion
Our results suggested that mRNA vaccination was well tolerated and effective in frail and disabled nursing home residents, most of whom retained positive serology after 6 months of follow-up for the Delta variant B.1.617.2 COVID-19.
Old age, dementia, disability, frailty, and high comorbidity have been linked to elevated COVID-19 mortality among LTCF residents [12, 13], and it was initially thought that these factors may predispose to a worse response to vaccination as well as a greater number of ADR effects, including mortality [6]. Thus, both safety and efficacy of vaccinations in older people are critical to their success [14]. Although care home residents are likely to be among the highest risk groups and were the first group to be vaccinated in Spain [15], they are usually excluded from clinical trials without taking into account geriatric conditioning variables [14, 16].
The sample we used in our study was representative of the most vulnerable population in society (nursing home residents), whose immunological characteristics and clinical situation differ from those of the rest of the population and which have given rise to greater concerns about safety. It has to be highlighted that the high participation rate in this study—98.6% of all residents—might have been due to the collaborative work of the LTCF with the Liaison Geriatrician, a figure created at institutional level by the Madrid government during the pandemic with the aim of strengthening health care and improving coordination between residential/nursing homes and hospitals. In the case of our hospital, the Liaison Geriatrician has been in place since 2012, providing direct hospital support to the nursing home’s medical team [17, 18].
The ADRs in our elderly population, with a high grade of frailty, were not higher than those observed in other studies conducted with real-life data in populations over 55 years of age [19, 20]. Even in patients with palliative care needs, the vaccine was well tolerated, with only 10% of these patients having developed ADRs, of which none were severe. ADRs in our sample were less frequent than in the BNT162b2 clinical trial, however a higher number of severe adverse reactions were recorded [4]. On the other hand, 70.8% of participants in our study had previously been ill with COVID-19, compared with the population assessed in the BNT162b2 trial, where subjects with a history of COVID-19 infection were excluded. Therefore, we concluded that the only variable that showed an association with having more severe adverse reactions was the existence of a previous positive serology. Having had a previous COVID-19 infection was the strongest predictor of anti-spike antibody titers at 3 and 6 months [21] and this would justify the higher frequency of severe reactions observed in our sample, compared with the results obtained in other studies [22].
In our study, the results of the individuals with previous SARS-CoV-2 infection who had positive serology prior to vaccination were similar to the results obtained regarding the accuracy and specificity of the diagnostic tests from the data of the seroprevalence study carried out in LTCFs in the Madrid community. It has to be mentioned that our nursing home under study was in the upper range of SARS-CoV-2 infection during the first pandemic wave [23, 24]. In older nursing home residents, SARS-CoV-2 infection history was the strongest predictor of anti-spike antibody titers at 6 months, whereas age and frailty were independently associated with lower titers at 6 months [21]. The persistence of IgG after vaccination in nursing home residents is high (95.2%) and was similar to the rate found in our study [16], regardless of the degree of frailty [25].
The most important results of our study were the long-term persistence of immunogenicity, with a mean follow-up of 7.7 months, and the differences observed according to age, irrespective of functional or cognitive status and degree of frailty. In our trial, even those with a need for palliative care preserved their serology (Table 3). A limitation of these data is that a qualitative and not a quantitative antibody detection test was used. With antibody quantification, various studies verified that although the antibodies are present, there is a decrease in these antibodies. Pre-vaccination COVID-19 illness was the only factor associated with a lower rate of antibody decline at 6 months, while frailty, disability, older age, cognitive impairment, or comorbidity were not associated with the extent of antibody loss [26, 27].
Finally, our complete vaccination effectiveness data also agree with data from similar studies [19, 28]. Factors that may influence the effectiveness of the vaccine and that should be taken into account include the cumulative incidence in 14 days (during the study, this varied between 1041 and 46 cases diagnosed per 100,000 inhabitants in the region of Madrid), the apparition of new SARS-CoV-2 strains, or the implementation of other measures such as social distancing, hand hygiene, or obligatory use of masks. It is possible that effectiveness data may vary depending on other circulating variants, relaxation of non-pharmacological measures, or even with the vaccination rate in each country.
The limitations of this study are mainly the size of the sample and the fact that this was not a multicenter study. The small sample size has been an important limitation in the assessment of the effectiveness of vaccination against SARS-CoV-2 infection. Even so, this study provided results in line with the scientific evidence available to date in nursing home residents [1, 28]. In safety terms, long-term safety was not assessed and only the ADRs produced after administration of the first and second doses were recorded. In addition, the algorithm used to assess causality has some limitations when evaluating the effect of drug withdrawal and the effect of re-exposure, due to the administration of the vaccine in two single doses. In the measurement of re-exposure, a more definite causality was given to reactions that only occurred after the second dose, and a lesser causality was given to reactions that only occurred after the first administration.
5 Conclusion
The BNT162B2 vaccine is well tolerated and effective in nursing home residents, independently of their clinical, comorbidity, functional, cognitive, or frailty characteristics. For the most part, immunogenicity has been maintained over time, regardless of the geriatric status of the participants. Our results support the few studies carried out regarding the vaccine’s safety and efficacy in nursing homes, emphasizing the importance of adequate vaccination in all residents, thus lowering the risk of worst outcome of the SARS-CoV-2 infection even with more benign variants.
References
Mazagatos C, Monge S, Olmedo C, et al. Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalizations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Euro Surveil. 2021;26(24):2100452. https://doi.org/10.2807/1560-7917.ES.2021.26.24.2100452.
Andrew MK, McElhaney JE. Age and frailty in COVID-19 vaccine development. Lancet. 2021;396(10267):1942–4. https://doi.org/10.1016/S0140-6736(20)32481-8.
McElhaney JE, Taddio A, Harmon SHE. The elderly, the frail, and COVID-19 vaccines: what we know so far; 2021. Royal Society of Canada. https://rsc-src.ca/en/voices/elderly-frail-and-covid-19-vaccines-what-we-know-so-far. Accessed Dec 2021.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577.
Agencia española de medicamentos y productos sanitarios (2021). 9º Informe de Farmacovigilancia sobre Vacunas COVID-19. https://www.aemps.gob.es/laAEMPS/docs/informe-farmacovigilancia-octubre-2021.pdf?x57618. Accessed 31 Oct 2021.
Torjesen I. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ. 2021;372: n149. https://doi.org/10.1136/bmj.n149.
Theou O, Pérez-Zepeda MU, van der Valk AM, Searle SD, Howlett SE, Rockwood K. A classification tree to assist with routine scoring of the Clinical Frailty Scale. Age Ageing. 2021;50(4):1406–11. https://doi.org/10.1093/ageing/afab006.
Torné A, Puigoriol E, Zabaleta-Del-Olmo E, Zamora-Sánchez JJ, Santaeugènia S, Amblàs-Novellas J. Reliability, validity, and feasibility of the Frail-VIG Index. Int J Environ Res Public Health. 2021;18(10):5187. https://doi.org/10.3390/ijerph18105187.
Langa LS, Sallent LV, Díez SR. Interpretación de las pruebas diagnósticas de la COVID-19. FMC. 2021;28(3):167–73. https://doi.org/10.1016/j.fmc.2021.01.005.
US FDA. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Rockville: Center for Biologics Evaluation and Research; 2007.
Aguirre C, García M. Evaluación de la causalidad en las comunicaciones de reacciones adversas a medicamentos. Algoritmo del Sistema Español de Farmacovigilancia [Causality assessment in reports on adverse drug reactions. Algorithm of Spanish pharmacovigilance system]. Med Clin (Barc). 2016;147(10):461–4. https://doi.org/10.1016/j.medcli.2016.06.012.
Lozano-Montoya I, Quezada-Feijoo M, Jaramillo-Hidalgo J, et al. Mortality risk factors in a Spanish cohort of oldest-old patients hospitalized with COVID-19 in an acute geriatric unit: the OCTA-COVID study. Eur Geriatr Med. 2021;12(6):1169-1180. doi: https://doi.org/10.1007/s41999-021-00541-0.
Levin AT, Jylhävä J, Religa D, Shallcross L. COVID-19 prevalence and mortality in longer-term care facilities. Eur J Epidemiol. 2022;37(3):227–234. https://doi.org/10.1007/s10654-022-00861-w.
Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50(2):279–83. https://doi.org/10.1093/ageing/afaa274.
Grupo de trabajo vacunación en población adulta y grupos de riesgo de la Ponencia de Programa y Registro de Vacunaciones. Vacunación en grupos de riesgo de todas las edades y en determinadas situaciones. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad, Consumo y Bienestar Social, julio 2018. https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/riesgo/docs/VacGruposRiesgo_todas_las_edades.pdf. Accessed 31 Jan 2022.
Andrew MK, Schmader KE, Rockwood K, Clarke B, McElhaney JE. Considering frailty in SARS-CoV-2 vaccine development: how geriatricians can assist. Clin Interv Aging. 2021;16:731–8. https://doi.org/10.2147/CIA.S295522.
Menéndez-Colino R, Argentina F, de Miguel AM, Barcons Marqués M, Chaparro Jiménez B, Figueroa Poblete C, et al. La Geriatría de Enlace con residencias en la época de la COVID-19. Un nuevo modelo de coordinación que ha llegado para quedarse [Liaison geriatrics with nursing homes in COVID time. A new coordination model arrived to stay]. Rev Esp Geriatr Gerontol. 2021;56(3):157–65. https://doi.org/10.1016/j.regg.2021.01.002.
González-Montalvo JI, Ramírez-Martín R, Menéndez Colino R, Alarcón T, Tarazona-Santabalbina FJ, Martínez-Velilla N, Vidán MT, et al. Cross-speciality geriatrics: a health-care challenge for the 21st century. Rev Esp Geriatr Gerontol. 2020;55(2):84–97. https://doi.org/10.1016/j.regg.2019.10.011.
Cai C, Peng Y, Shen E, et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol Ther. 2021;29(9):2794–805. https://doi.org/10.1016/j.ymthe.2021.08.001.
Ripabelli G, Sammarco ML, Rezza G, et al. A SARS-CoV-2 outbreak among nursing home residents vaccinated with a booster dose of mRNA COVID-19 vaccine. J Community Health. 2022. https://doi.org/10.1007/s10900-022-01082-8.
Dyer AH, Noonan C, McElheron M, et al. Previous SARS-CoV-2 infection, age, and frailty are associated with 6-month vaccine-induced anti-spike antibody titer in nursing home residents. J Am Med Dir Assoc. 2022;23(3):434–9. https://doi.org/10.1016/j.jamda.2021.12.001.
Baldolli A, Michon J, Appia F, et al. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: a case control study. Vaccine. 2021;39(32):4410–3. https://doi.org/10.1016/j.vaccine.2021.06.054.
Böger B, Fachi MM, Vilhena RO, Cobre AF, Tonin FS, Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control. 2021;49(1):21–9. https://doi.org/10.1016/j.ajic.2020.07.011.
Candel FJ, Barreiro P, SanRomán J, Del Mar Carretero M, Sanz JC, Pérez-Abeledo M, Ramos B, Investigators of the SeroSOS study, et al. The demography and characteristics of SARS-CoV-2 seropositive residents and staff of nursing homes for older adults in the Community of Madrid: the SeroSOS study. Age Ageing. 2021;50(4):1038–47. https://doi.org/10.1093/ageing/afab096.
Salmerón Ríos S, Mas Romero M, Cortés Zamora EB, et al. Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study. J Am Geriatr Soc. 2021;69(6):1441–7. https://doi.org/10.1111/jgs.17153.
Seiffert P, Konka A, Kasperczyk J, et al. Immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in older residents of a long-term care facility: relation with age, frailty and prior infection status. Biogerontology. 2022;23(1):53–64. https://doi.org/10.1007/s10522-021-09944-9.
Salmerón Ríos S, Cortés Zamora EB, Avendaño Céspedes A, et al. Immunogenicity after 6 months of BNT162b2 vaccination in frail or disabled nursing home residents: the COVID-A Study. J Am Geriatr Soc. 2022;70(3):650–8. https://doi.org/10.1111/jgs.17620.
Tenforde MW, Olson SM, Self WH, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):674–9. https://doi.org/10.15585/mmwr.mm7018e1.
Acknowledgements
This research was carried out using the extraordinary fund of initiatives “UAX-Santander COVID-19”, a resource of Alfonso X el Sabio University, as part of registered project 1.011.103.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this study was provided by Alfonso X el Sabio University.
Conflict of interest
Pablo Montejano-Hervás, Javier Gómez-Pavón, Olga Tornero-Torres, Mª Victoria Valverde-Moyar, Beatriz Martín Cruz, Maribel Vela Carbonera, Raquel Fuentes-Irigoyen, Pilar Tejada González, Margarita González-Becerra, Esther Higueras Sánchez, and Primitivo Ramos Cordero have no conflicts of interest to declare.
Author contributions
Study design: JGP, OTT, RFI. Data collection: MVVM, BMC, MVC, MGB, EHS. Data analysis: JGP, OTT, PMH, RFI, PTG. Drafting of the manuscript: PMH, JGP. Critical revision of the manuscript: All authors.
Ethics approval
This study was approved by the local Research Ethics Committee (registration code PI-4616) and was conducted in accordance with the Declaration of Helsinki.
Consent to participate
All participants signed the informed consent form.
Consent for publication
Not applicable.
Availability of data
Anonymized data are available from the corresponding author upon request.
Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Montejano-Hervás, P., Gómez-Pavón, J., Tornero-Torres, O. et al. Safety, Effectiveness, and Immunogenicity 6 Months After BNT162B2 mRNA Vaccine in Frail Nursing Home Residents. Drugs Aging 39, 587–595 (2022). https://doi.org/10.1007/s40266-022-00959-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-022-00959-6