Abstract
Treatment for the eradication of Helicobacter pylori infection, a leading cause of peptic ulcer disease and an important risk factor for gastric cancer and mucosa-associated lymphoid tissue lymphoma, is indicated whenever infection is identified. However, treatment success rates with current guideline-recommended proton-pump inhibitor (PPI)-based regimens remain suboptimal, with one potential factor associated with treatment failure being inadequate acid suppression. Vonoprazan (Voquezna®) is a first-in-class potassium-competitive acid blocker with the potential to provide potent and sustained acid suppression. Following clinical trials conducted mainly in Asia (supported by post-marketing experience from Asia) and the phase III PHALCON-HP trial conducted in the USA and Europe, vonoprazan is now approved in the USA for use in combination with amoxicillin (dual therapy) or amoxicillin and clarithromycin (triple therapy) for the treatment of H. pylori infection in adults. The vonoprazan-based dual and triple therapy regimens were generally well tolerated in PHALCON-HP. In addition, vonoprazan has advantages including a rapid onset of action and no food effect, making vonoprazan-based dual and triple therapy regimens valuable alternatives to standard PPI-based triple therapy in the treatment of H. pylori infection.
Plain Language Summary
Infection with the bacterium Helicobacter pylori is a leading cause of peptic ulcer disease and has been identified as an important risk factor for gastric cancer. Current recommended treatments for H. pylori infection generally involve a combination of antibiotics together with an acid suppressant, such as a proton-pump inhibitor (PPI). However, treatment success rates with current guideline-recommended PPI-based regimens remain suboptimal. Vonoprazan (Voquezna®), from a new class of drugs known as potassium-competitive acid blockers, has the potential to provide potent and sustained acid suppression. Based on the findings of a pivotal trial (PHALCON-HP) conducted in the USA and Europe, vonoprazan is now approved in the USA for the treatment of H. pylori infection in adults when used in combination with amoxicillin, or amoxicillin and clarithromycin. Alongside the demonstrated efficacy and tolerability of the vonoprazan-based regimens, vonoprazan has a rapid onset of action and can be taken with or without food. Thus, vonoprazan-based dual and triple therapy regimens present valuable alternatives to standard PPI-based triple therapy in the treatment of H. pylori infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.24862875. |
Vonoprazan-based dual and triple therapy regimens are non-inferior to lansoprazole-based triple therapy in patients infected with H. pylori strains not resistant to clarithromycin or amoxicillin, based on eradication rates in the PHALCON-HP trial |
Additionally, the vonoprazan-based regimens are superior to lansoprazole-based triple therapy in patients who have a clarithromycin-resistant strain of H. pylori and in the overall population, based on PHALCON-HP |
1 Introduction
Helicobacter pylori infection, one of the most common chronic bacterial infections worldwide, is a leading cause of peptic ulcer disease [1, 2]. Furthermore, with H. pylori infection being recognized as an important risk factor for gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma, it is recommended that H. pylori be eradicated whenever infection is identified [1, 2]. Recommended first-line treatment options are bismuth-based quadruple therapy [typically consisting of a proton-pump inhibitor (PPI), bismuth, tetracycline, and metronidazole] for 10–14 days or triple therapy consisting of a PPI, clarithromycin, and amoxicillin or metronidazole for 14 days [1, 2]. However, treatment success rates remain suboptimal, with these regimens (particularly the triple therapy regimens) commonly associated with eradication rates in the USA of ≤ 80% [3]. While antibiotic resistance and patient nonadherence to treatment are the most common reasons for H. pylori eradication failure [1, 3, 4], inadequate acid suppression has also been associated with treatment failure [4]. In particular, nocturnal acid breakthrough is a concern, with the duration of nocturnal acid breakthrough found to significantly influence H. pylori eradication rates [5].
Studies investigating the role of acid suppression in the treatment of H. pylori infection have indicated that profound and sustained acid suppression is critical for successful treatment [6, 7]. One study found that the time intragastric pH was above pH 4 and the achievement of prolonged (continuous) periods with pH > 6 were significantly associated with higher eradication rates [6]. Another study, in patients treated with a PPI-based triple therapy regimen, found that the median 24-h pH was significantly higher in patients with successful eradication than in patients without eradication [7]. Given these findings, more effective acid suppression has been targeted as a potential way to improve eradication rates in the treatment of H. pylori infection [8, 9]. Within this context has come the development of potassium-competitive acid blockers (PCABs) and evidence that agents in this drug class are associated with more potent and sustained acid suppression relative to PPIs [9,10,11,12].
Vonoprazan (Voquezna®), the first-in-class PCAB, has been evaluated as part of combination regimens for the treatment of H. pylori infection and received its first approval in 2014, in Japan [13]. Based on the findings of the randomized, active-controlled, phase III trial PHALCON-HP trial conducted in the USA and Europe [14], and following a reformulation to address an issue concerning trace levels of nitrosamine impurities detected in early commercial batches, vonoprazan is now approved in the USA for use in combination with amoxicillin, or amoxicillin and clarithromycin, for the treatment of H. pylori infection in adults [15, 16]. This article reviews data on the efficacy, safety, and tolerability of these vonoprazan-based triple and dual therapy regimens in the treatment of H. pylori infection in adults, with a focus on the US label. Pharmacologic data for vonoprazan are also summarized. Vonoprazan has also been approved in the USA for use (as monotherapy) for healing and maintenance of healing and relief of heartburn associated with all grades of erosive esophagitis in adults [16] and in other parts of the world for other acid-related disorders; however, discussion of the use of vonoprazan in these other indications is beyond the scope of this article.
2 Pharmacodynamic Properties of Vonoprazan
Vonoprazan is a potent inhibitor of the gastric proton pump H+/K+-ATPase [16, 17]. It binds to H+/K+-ATPase in a noncovalent and reversible manner, blocking potassium binding through competitive inhibition. Through this action, vonoprazan causes rapid, profound, and sustained suppression of gastric acid secretion, resulting in an elevation in intragastric pH [18]. The increase in intragastric pH improves the stability of antibacterials used in the treatment of H. pylori infection [8, 15]. In addition, the elevated intragastric pH helps to promote H. pylori bacterial replication, increasing the susceptibility of the bacteria to antibacterial agents which require active bacterial growth for optimal effectiveness [15, 19]. Of note, there is evidence that an external pH in the narrow range of pH 6 to pH 7 is required for optimal sensitivity of H. pylori to growth-dependent antibiotics such as amoxicillin, clarithromycin, and tetracycline [20].
Vonoprazan binds H+/K+-ATPase with high affinity, with reported Ki values of 10 nmol/L [17] and 3 nmol/L [21] at pH 7 and pH 6.5, respectively. Furthermore, vonoprazan is able to bind the H+/K+-ATPase both when the enzyme is in an active or inactive state [22]. Acid suppression caused by vonoprazan is dose-dependent, based on two randomized, double-blind, placebo-controlled phase I trials conducted in Japan and in the UK in healthy male subjects who received vonoprazan 10–40 mg once daily for 7 days [18]. Pharmacodynamic data were similar in the Japanese and UK trials. Elevations in intragastric pH had a rapid onset (occurring within 2–3 h after the first dose) and were maintained through the 24-h dosing interval. On day 7, mean 24-h intragastric pH > 5 holding time ratios (HTRs) with vonoprazan 20 mg and 40 mg, respectively, were 73.2% and 98.6% in the Japanese study and 78.6% and 85.0% in the UK study. Furthermore, mean night-time pH > 5 HTRs on day 7 with the respective doses were 55.9% and 97.2% in the Japanese study and 66.9% and 77.5% in the UK study [18]. In a separate study in healthy US subjects, the 24-h intragastric pH > 6 HTR on day 7 was significantly (p < 0.0001) higher with vonoprazan 20 mg once daily (62.5%) than with lansoprazole 30 mg once daily (16.4%) [12].
3 Pharmacokinetic Properties of Vonoprazan
Vonoprazan has time-independent pharmacokinetics [16, 18]. Following oral administration, vonoprazan is rapidly absorbed, reaching maximum plasma concentrations approximately 1–3 h post dosing. Based on clinical trials with once-daily dosing in healthy subjects, vonoprazan exposure is approximately dose-proportional over the dose range of 10–40 mg, with steady-state concentrations reached by day 3–4 [16, 18]. There is no clinically relevant food effect on vonoprazan pharmacokinetics [23], and the drug can be taken without regard to food [15, 16]. With twice-daily dosing of vonoprazan 20 mg, mean steady-state plasma exposure is approximately 1.8-fold higher than at day 1 [16]. At steady state with twice daily dosing, vonoprazan has an apparent oral volume of distribution of 782.7 L. Plasma protein binding of vonoprazan is approximately 85–88% [16].
Vonoprazan undergoes extensive metabolism, with the resulting metabolites having no pharmacologic activity [16]. Based on in vitro studies, vonoprazan metabolism is primarily mediated by CYP3A4, with CYP2B6, CYP2C9, CYP2C19, CYP2D6, and sulfo- and glucuronosyl-transferases also involved [16, 24]. Vonoprazan has an elimination half-life of approximately 7 h [16]. Apparent oral clearance at steady state with twice-daily dosing is 81.3 L/h. Following oral administration of a radiolabeled dose of vonoprazan, approximately 67% and 31% of the dose was recovered in urine and feces, respectively, mostly as metabolites [16].
There were no clinically significant differences in vonoprazan pharmacokinetics based on sex, age (< 65 vs ≥ 65 years), race (Asian vs non-Asian), or CYP2C19 metabolizer status [16, 18, 25, 26].
3.1 Potential Drug Interactions
In an open-label, randomized, four-way crossover phase I study in healthy male Japanese subjects, twice-daily administration of vonoprazan 20 mg with amoxicillin 750 mg and clarithromycin 400 mg was associated with an 85% increase in vonoprazan exposure and a 45% increase in clarithromycin exposure [based on area under the plasma concentration-time curve (AUC) from time 0 to 12 h (AUC12h), but with no effect on amoxicillin exposure, compared with administration of each component alone [27].
Noting the metabolism of vonoprazan by CYP3A4 (Sect. 3 above), strong or moderate CYP3A inducers may decrease exposure of vonoprazan, potentially reducing its efficacy [15, 16], with modeling suggesting an approximately 80% lower vonoprazan exposure when co-administered with a strong CYP3A4 inducer (e.g., rifampicin) and an approximately 50% lower vonoprazan exposure when administered with a moderate CYP3A4 inducer (e.g., efavirenz) [28]; it is thus recommended that concomitant use of strong or moderate CYP3A inducers with vonoprazan be avoided [16]. Additionally, co-administration of CYP3A inhibitors and vonoprazan has the potential to increase vonoprazan exposure [15], as was observed in the phase I study discussed above with co-administration of vonoprazan with amoxicillin and clarithromycin (a strong CYP3A inhibitor) [27]. Similarly, in an open-label, sequential design phase I study in healthy male subjects, a single dose of vonoprazan 40 mg administered with clarithromycin 500 mg twice daily for 7 days was associated with a 58% increase in vonoprazan AUC from time 0 to infinity (AUC∞) compared with vonoprazan administered alone [29].
As a weak inhibitor of CYP3A, vonoprazan may also increase the exposure of drugs metabolized by CYP3A4, potentially increasing the risk of adverse events associated with these drugs [15, 16, 30]. Furthermore, the potential significance of this interaction is likely to be heightened with co-administration of clarithromycin (a strong CYP3A inhibitor), for example with use of vonoprazan, amoxicillin, and clarithromycin triple therapy [15].
Based on in vitro studies, vonoprazan is a CYP2C19 inhibitor [15, 16]. Concomitant use of vonoprazan with drugs that are substrates of CYP2C19 may affect the exposure of the co-administered drugs [15, 16]. For example, co-administration of vonoprazan with clopidogrel, which is metabolized to its active metabolite in part by CYP2C19, may reduce plasma concentrations of the clopidogrel active metabolite, reducing its efficacy in platelet inhibition. Similarly, co-administration with vonoprazan may increase exposure of drugs that are substrates of CYP2C19 (e.g., citalopram, cilostazol) [15, 16].
Due to its effects on intragastric pH (Sect. 2), vonoprazan may cause alterations in the exposure of drugs that are dependent on gastric pH for absorption (e.g., rilpivirine, atazanavir, nelfinavir, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole, itraconazole), potentially affecting their safety and/or effectiveness [15, 16]. The reduction in intragastric acidity caused by vonoprazan also leads to increases in chromogranin A levels, which could cause false positive results in diagnostic tests for neuroendocrine tumors [15, 16].
4 Therapeutic Efficacy of Vonoprazan
Early clinical trial data supporting the efficacy of vonoprazan-based regimens in the treatment of H. pylori infection were derived from trials performed in Japan [31,32,33,34,35,36], including a randomized, double-blind, phase III trial which assessed the efficacy of a vonoprazan, amoxicillin, and clarithromycin regimen versus lansoprazole, amoxicillin, and clarithromycin triple therapy in 650 H. pylori-positive patients with a history of gastric or duodenal ulcers [32]. To support regulatory assessment by the US FDA, the efficacy of vonoprazan-based dual and triple therapy regimens in the treatment of H. pylori infection was subsequently evaluated in the randomized, active-controlled, phase III PHALCON-HP trial, conducted in the USA and Europe [14]. Given the focus of this article on the use of vonoprazan according to the US label (including use with the approved drug combinations, doses, and treatment duration) (Sect. 6), this section focuses on the PHALCON-HP trial.
4.1 PHALCON-HP Trial
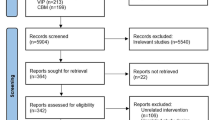
The PHALCON-HP trial design is summarized in Fig. 1. In the trial, 1046 treatment-naïve adults with H. pylori infection were randomized 1 ꞉ 1 ꞉ 1 to open-label vonoprazan dual therapy (vonoprazan 20 mg twice daily plus amoxicillin 1000 mg three times daily), or double-blind vonoprazan or lansoprazole triple therapy (vonoprazan 20 mg or lansoprazole 30 mg, plus amoxicillin 1000 mg and clarithromycin 500 mg) twice daily, with all study drugs administered orally for 14 days [14]. Eligible patients had at least one of the following clinical conditions: dyspepsia lasting ≥ 2 weeks; functional dyspepsia; a recent/new diagnosis of nonbleeding peptic ulcer; history of peptic ulcer not previously treated for H. pylori infection; or requirement for long-term, nonsteroidal, anti-inflammatory drug treatment at a stable dose. The presence of H. pylori infection was confirmed at screening by a positive 13C-urea breath test (UBT). Exclusion criteria included: gastric cancer; gastric or duodenal ulcer with current or recent bleeding; or clinically significant gastrointestinal bleeding within the 4 weeks preceding randomization [14].
Trial design of the randomized, active-controlled, multicenter, phase III PHALCON-HP trial in treatment-naïve adults with H. pylori infection [14] with further information available in Table 1. Efficacy results are reported in the animated figure (available online). BGD between-group difference, pts patients *Primary and secondary endpoints assessed at week 6 (4 weeks after the last dose of study drug), although due to operational challenges caused by the Covid-19 pandemic, results obtained between 27 and 141 days after the last dose were permitted to be included in the full analysis set. **Among patients who, at baseline, were infected with H. pylori strains that were not resistant to clarithromycin or amoxicillin
(MP4 8939 KB)
In total, 992 patients (94.8% of all randomized patients) were included in the full analysis set (FAS), composed of all randomized patients who had H. pylori infection confirmed by histology and/or culture from a biopsy taken at baseline after a positive 13C-UBT at screening [14]. Demographics and baseline characteristics were well balanced across the three treatment groups. In the FAS, 41.5% of patients were from the USA and 58.5% were from Europe (Bulgaria, Czech Republic, Hungary, Poland, and the UK). For the vast majority (98.0%) of patients, the qualifying clinical condition was dyspepsia (either dyspepsia lasting ≥ 2 weeks or confirmed functional dyspepsia). Antibiotic resistance data were available for 293, 308, and 306 patients in the vonoprazan dual therapy, triple therapy, and lansoprazole triple therapy groups, respectively. Across the three groups, 19.1–23.7% of patients had H. pylori strains that were resistant to clarithromycin, 1.0–1.6% had strains that were resistant to amoxicillin, and 67.2–71.2% had strains that were resistant to metronidazole (with some patients having strains that were resistant to more than one antibiotic). For efficacy analyses, patients without antibiotic resistance data were classed as having strains that were not resistant to clarithromycin and amoxicillin [14].
Efficacy endpoints in PHALCON-HP centered on the H. pylori eradication rate [14]. Eradication was based on a negative 13C-UBT at test-of cure, with different endpoints evaluating the treatment effect in different analysis populations. Under the original protocol, the test-of-cure visit was set to be at week 6 (i.e., 4 weeks after the last dose of study drugs). However, due to extensive operational challenges caused by the COVID-19 global pandemic, the test-of-cure visit window was modified, with data from 27–141 days after the last dose of study drugs permitted for inclusion in the efficacy analyses. Patients without a test-of-cure 13C-UBT result were considered to be treatment failures (i.e., H. pylori infection not eradicated). Primary and secondary endpoints in PHALCON-HP were analyzed hierarchically. The primary endpoint was the H. pylori eradication rate among patients infected with H. pylori strains at baseline that were not resistant to clarithromycin or amoxicillin, with assessment of the non-inferiority of the vonoprazan regimens to lansoprazole triple therapy with a non-inferiority margin of 10%. Predefined secondary endpoints evaluated the H. pylori eradication rate firstly in patients with a clarithromycin-resistant strain at baseline and secondly in the overall (FAS) population, with assessment of the superiority of the vonoprazan regimens to lansoprazole triple therapy [14].
The primary endpoint was met in the PHALCON-HP trial, with the vonoprazan dual and triple therapy regimens both being non-inferior to lansoprazole triple therapy with regard to H. pylori eradication rates in patients with strains that were not resistant to clarithromycin or amoxicillin (Table 1) [14]. In these patients, H. pylori eradication rates with vonoprazan dual therapy, vonoprazan triple therapy, and lansoprazole triple therapy, respectively, were 78.5%, 84.7%, and 78.8%; corresponding rates for the subset of patients at US sites were 76.1%, 79.6%, and 78.4%. In the secondary endpoint analyses, the vonoprazan dual and triple therapy regimens were both superior to lansoprazole triple therapy based on H. pylori eradication rates, both in patients who had a clarithromycin-resistant H. pylori strain and in the overall (FAS) population (Table 1). In addition, analyses were performed where the primary and secondary endpoints were evaluated using the corresponding populations in the per-protocol set, with criteria for the per-protocol set including having taken ≥ 75% of each study drug and having a 13C-UBT result from a test-of -cure visit between 28 and 56 days after the end of treatment. The findings from these per-protocol analyses were consistent with the findings based on the FAS populations [14].
5 Tolerability of Vonoprazan
Vonoprazan, used in combination with amoxicillin with or without clarithromycin, is generally well tolerated in adults with H. pylori infection. In the PHALCON-HP trial (Sect. 4), treatment-emergent adverse events (TEAEs) were reported in 29.9%, 34.1%, and 34.5% of patients in the vonoprazan dual therapy, vonoprazan triple therapy, and lansoprazole triple therapy groups, respectively [14]. In all groups, most TEAEs were mild to moderate in severity. TEAEs occurring in ≥ 2% of patients in either of the vonoprazan-based therapy groups included diarrhea, dysgeusia, headache, mycotic vulvovaginal infection, SAR-CoV-2 infection, hypertension, and nasopharyngitis (Fig. 2). Serious TEAEs were uncommon, occurring in 1.4%, 1.7%, and 0.9% of patients in the vonoprazan dual therapy, vonoprazan triple therapy, and lansoprazole triple therapy groups, respectively; no serious TEAEs were considered to be treatment related. In the respective groups, three (0.9%), eight (2.3%), and four (1.2%) patients experienced TEAEs leading to treatment discontinuation. Three patients died during the trial, one in each of the vonoprazan and lansoprazole triple therapy groups due to COVID-19, and one in the vonoprazan triple therapy group from sudden cardiac arrest [14].
Treatment-emergent adverse events occurring in ≥ 2% of patients in any treatment group in the phase III PHALCON-HP trial [14].
Acute tubulointerstitial nephritis has also been reported during treatment with vonoprazan [16]. Other adverse events that have been reported in post-marketing surveillance in patients receiving vonoprazan (outside of the USA) include anaphylactic shock, urticaria, drug eruption, hepatic injury, hepatic failure, jaundice, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis; however the frequency of such events or any causal relationship to vonoprazan has not been established [15].
6 Dosage and Administration of Vonoprazan
Vonoprazan is approved in the USA for use in combination with amoxicillin, or amoxicillin and clarithromycin, for the treatment of H. pylori infection in adults [15, 16]. The recommended dosage for the dual therapy regimen is vonoprazan 20 mg twice daily and amoxicillin 1000 mg three times daily for 14 days. The recommended dosage for the triple therapy regimen is vonoprazan 20 mg, amoxicillin 1000 mg, and clarithromycin 500 mg, each given twice daily for 14 days. Both the dual and triple therapy regimens are also available as US FDA-approved co-packaged products (Voquezna® Dual Pak® and Voquezna® Triple Pak®, respectively), with the drugs supplied with the recommended dosages [15]. For both regimens, all components are to be taken orally and can be taken with or without food [15, 16]. No dosage adjustments are recommended for either regimen in patients with mild to moderate renal impairment (estimated GFR ≥ 30 mL/min) or mild hepatic impairment (Child-Pugh Class A). Neither regimen should be used in patients with severe renal impairment (estimated GFR < 30 mL/min) or with moderate to severe hepatic impairment (Child-Pugh Class B or C). Breastfeeding is not recommended during treatment with either of the vonoprazan-based regimens [15, 16], although breast milk can be pumped and discarded during and for 2 days after treatment with either regimen [15].
Both regimens are contraindicated with concomitant use of rilpivirine-containing products, and in patients with a known hypersensitivity to vonoprazan, amoxicillin, or other β-lactam antibacterials; the triple therapy regimen is additionally contraindicated in patients with a known hypersensitivity to clarithromycin or other macrolide antibacterials [15, 16].
Local prescribing information should be consulted for full details on the administration of the vonoprazan-based dual and triple therapy regimens, including further information on warnings and precautions, potential adverse reactions, potential drug interactions, use in specific populations, and contraindications associated with the antibacterial components of the regimens.
7 Place of Vonoprazan in the Management of H. pylori Infection
Noting the established association of H. pylori infection with serious disease (including gastric cancer and MALT lymphoma), there is consensus that treatment targeting H. pylori eradication is indicated in all patients in whom active H. pylori infection is identified (Sect. 1) [1, 2].
The efficacy of vonoprazan-based regimens in the treatment of H. pylori infection has been demonstrated across a range of clinical trials conducted in Japan [31,32,33,34,35,36] and other parts of Asia [37,38,39,40,41]. Furthermore, several meta-analyses have been performed (primarily based on data from studies in Asia), generally showing favorable eradication rates for vonoprazan-based over PPI-based regimens [42,43,44,45,46,47]. The PHALCON-HP trial (Sect. 4.1) was conducted to further evaluate the efficacy of two vonoprazan-based regimens in the treatment of H. pylori infection in Western populations, with the aim to support regulatory assessment by the US FDA, in particular noting that factors such as differences in antibiotic resistance rates across geographic areas as well as potential differences in drug pharmacokinetics across different populations have the potential to influence trial findings and risk-benefit assessments [1, 2]. In the PHALCON-HP trial, both the vonoprazan-based dual and triple therapy regimens were non-inferior to PPI-based triple therapy in patients infected with H. pylori strains that were not resistant to clarithromycin or amoxicillin and were superior to PPI-based triple therapy in patients with clarithromycin-resistant strains and in the overall study population [14]. Although superior to the PPI-based triple therapy regimen in the overall population, the eradication rates with the vonoprazan-based regimens remained below 90% [14], a threshold which is considered a target for empirical treatment [48]. Indeed, one limitation of the PHALCON-HP trial was that the comparator regimen (i.e., lansoprazole-based triple therapy) is not considered the standard-of-care when susceptibility information is unavailable or when clarithromycin resistance has been identified, with a bismuth-based quadruple therapy regimen generally recommended in these circumstances [2, 49]. Despite this limitation, and accepting that the trial was primarily designed to assess the impact of the acid suppression component of the regimen (i.e., vonoprazan versus the PPI lansoprazole), the superiority of the vonoprazan-based regimens over the PPI-based triple therapy regimen in the overall trial population supports the proposition that the enhanced gastric acid suppression provided by vonoprazan can improve the effectiveness of antibiotics used for H. pylori eradication [14].
While noting that the PHALCON-HP trial was not designed to compare the vonoprazan-based dual and triple therapy regimens with each other, the two vonoprazan-based regimens displayed similar eradication rates in all key efficacy outcomes investigated in the trial (Table 1), suggesting that using dual therapy (with high-dose amoxicillin) over triple therapy may be appropriate in some situations. Further study will be required to more precisely determine the relative roles of the vonoprazan-based dual and triple therapy regimens, although a dual therapy regimen (with high-dose amoxicillin) does have some potential advantages over triple (or quadruple) therapy regimens, including greater simplicity for patients, a lower potential for drug interactions and/or adverse events, and benefits in terms of antibiotic stewardship [36]. Indeed, one of the key potential benefits that the more effective acid suppression of PCABs over PPIs could provide is the possibility to achieve high eradication rates with simpler regimens (e.g., dual rather than triple therapy regimens) [9]. Further study in this area will be of interest.
Both of the US FDA-approved vonoprazan-based regimens for the treatment of H. pylori infection in adults (i.e., as were used in the PHALCON-HP trial) are 14-day regimens [15, 16], aligning with guideline recommendations supporting 14-day regimens over shorter courses, with evidence that longer courses are associated with higher treatment success rates [1, 2, 4]. The vonoprazan dual and triple therapy regimens were generally well tolerated in the PHALCON-HP trial (Sect. 5), with most TEAEs reported with the regimens being of mild to moderate severity [14]. There were no treatment-related serious TEAEs reported during the trial.
In summary, the demonstrated superiority of the vonoprazan-based regimens over the PPI-based triple therapy regimen in the overall (FAS) population in PHALCON-HP provides good support for a role for vonoprazan-based regimens in the treatment of H. pylori infection. Alongside the demonstrated efficacy and tolerability of the vonoprazan-based dual and triple therapy regimens, vonoprazan has several advantageous pharmacodynamic (Sect. 2) and/or pharmacokinetic (Sect. 3) properties. Vonoprazan has a rapid onset of action and provides potent and sustained acid suppression; its pharmacokinetics are not affected to any clinically relevant extent by CYP2C19 polymorphisms; and it can be taken without regard to food. In conclusion, collectively these findings suggest that vonoprazan-based dual and triple therapy regimens present valuable alternatives to standard PPI-based triple therapy in the treatment of H. pylori infection in adults.
Data Selection Vonoprazan: 291 records identified
Duplicates removed | 4 |
Excluded during initial screening (e.g., press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 150 |
Excluded during writing (e.g., reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 88 |
Cited efficacy/tolerability articles | 17 |
Cited articles not efficacy/tolerability | 32 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were: Vonoprazan, Takecab, TAK-438, Vonopion, Vonosap, Voquezna, Helicobacter pylori. Records were limited to those in English language. Searches last updated 18 Dec 2023. | |
Change history
13 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s40265-024-02042-3
References
Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–39.
Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71(9):1724–62.
Shah S, Hubscher E, Pelletier C, et al. Helicobacter pylori infection treatment in the United States: clinical consequences and costs of eradication treatment failure. Expert Rev Gastroenterol Hepatol. 2022;16(4):341–57.
Shah SC, Iyer PG, Moss SF. AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160(5):1831–41.
Kim JI, Park SH, Kim JK, et al. The effects of nocturnal acid breakthrough on Helicobacter pylori eradication. Helicobacter. 2002;7(6):331–6.
Sjöstedt S, Sagar M, Lindberg G, et al. Prolonged and profound acid inhibition is crucial in Helicobacter pylori treatment with a proton pump inhibitor combined with amoxicillin. Scand J Gastroenterol. 1998;33(1):39–43.
Sugimoto M, Furuta T, Shirai N, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12(4):317–23.
Scott DR, Sachs G, Marcus EA. The role of acid inhibition in Helicobacter pylori eradication. F1000Res. 2016;5(F1000 Faculty Rev):1747.
Scarpignato C, Hunt RH. Acid suppressant therapy: a step forward with potassium-competitive acid blockers. Curr Treat Options Gastro. 2021;19(1):94–132.
Ohkuma K, Iida H, Inoh Y, et al. Comparison of the early effects of vonoprazan, lansoprazole and famotidine on intragastric pH: a three-way crossover study. J Clin Biochem Nutr. 2018;63(1):80–3.
Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects—a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42(6):719–30.
Laine L, Sharma P, Mulford DJ, et al. Pharmacodynamics and pharmacokinetics of the potassium-competitive acid blocker vonoprazan and the proton pump inhibitor lansoprazole in US subjects. Am J Gastroenterol. 2022;117(7):1158–61.
Garnock-Jones KP. Vonoprazan: first global approval. Drugs. 2015;75(4):439–43.
Chey WD, Mégraud F, Laine L, et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: randomized clinical trial. Gastroenterology. 2022;163(3):608–19.
Phathom Pharmaceuticals Inc. Voquezna Triple Pak (vonoprazan tablets; amoxicillin capsules; clarithromycin tablets) and Voquezna Dual Pak (vonoprazan tablets; amoxicillin capsules): US prescribing information. 2023. https://www.phathompharma.com/wp-content/uploads/VOQUEZNA-TRIPLE-PAK-and-VOQUEZNA-DUAL-PAK-FDA-Final-Label-3.pdf. Accessed 19 Dec 2023.
Phathom Pharmaceuticals Inc. Voquezna (vonoprazan) tablets: US prescribing information. 2023. https://www.phathompharma.com/wp-content/uploads/VOQUEZNA-tablets-Prescriber-Information.pdf. Accessed 19 Dec 2023.
Shin JM, Inatomi N, Munson K, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H, K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther. 2011;339(2):412–20.
Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41(7):636–48.
Marcus EA, Inatomi N, Nagami GT, et al. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther. 2012;36(10):972–9.
Sachs G, Scott DR, Wen Y. Gastric infection by Helicobacter pylori. Curr Gastroenterol Rep. 2011;13(6):540–6.
Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335(1):231–8.
Scott DR, Munson KB, Marcus EA, et al. The binding selectivity of vonoprazan (TAK-438) to the gastric H+, K+-ATPase. Aliment Pharmacol Ther. 2015;42(11–12):1315–26.
Mulford DJ, Leifke E, Hibberd M, et al. The effect of food on the pharmacokinetics of the potassium-competitive acid blocker vonoprazan. Clin Pharmacol Drug Dev. 2022;11(2):278–84.
Yamasaki H, Kawaguchi N, Nonaka M, et al. In vitro metabolism of TAK-438, vonoprazan fumarate, a novel potassium-competitive acid blocker. Xenobiotica. 2017;47(12):1027–34.
Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol. 2015;6(6): e94.
Scarpignato C, Leifke E, Smith N, et al. A population pharmacokinetic model of vonoprazan: evaluating the effects of race, disease status, and other covariates on exposure. J Clin Pharmacol. 2022;62(6):801–11.
Sakurai Y, Shiino M, Okamoto H, et al. Pharmacokinetics and safety of triple therapy with vonoprazan, amoxicillin, and clarithromycin or metronidazole: a phase 1, open-label, randomized, crossover study. Adv Ther. 2016;33(9):1519–35.
Ramsden D, Mulford DJ, Zhang L, et al. Novel approach to evaluate the impact of moderate and strong CYP3A inducers on vonoprazan exposure [abstract Su1203 and poster]. Gastroenterology. 2022;162(7 suppl):S-544.
Jenkins H, Jenkins R, Patat A. Effect of multiple oral doses of the potent CYP3A4 inhibitor clarithromycin on the pharmacokinetics of a single oral dose of vonoprazan: a phase I, open-label, sequential design study. Clin Drug Investig. 2017;37(3):311–6.
Mulford DJ, Brudi P, Smith N, et al. A clinical drug interaction study to assess the effect of vonoprazan on the pharmacokinetics of midazolam [abstract Su1205 and poster]. Gastroenterology. 2022;162(7 suppl):S-545.
Maruyama M, Tanaka N, Kubota D, et al. Vonoprazan-based regimen is more useful than PPI-based one as a first-line Helicobacter pylori eradication: a randomized controlled trial. Can J Gastroenterol Hepatol. 2017;2017(4385161):1–7.
Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65(9):1439–46.
Sue S, Kuwashima H, Iwata Y, et al. The superiority of vonoprazan-based first-line triple therapy with clarithromycin: a prospective multi-center cohort study on Helicobacter pylori eradication. Intern Med. 2017;56(11):1277–85.
Sue S, Ogushi M, Arima I, et al. Vonoprazan- vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: a multicenter, prospective, randomized trial. Helicobacter. 2018;23(e12456):1–8.
Sue S, Shibata W, Sasaki T, et al. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J Gastroenterol Hepatol. 2019;34(4):686–92.
Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69(6):1019–26.
Hou X, Meng F, Wang J, et al. Vonoprazan non-inferior to lansoprazole in treating duodenal ulcer and eradicating Helicobacter pylori in Asian patients. J Gastroenterol Hepatol. 2022;37(7):1275–83.
Ang D, Koo SH, Chan YH, et al. Clinical trial: seven-day vonoprazan- versus 14-day proton pump inhibitor-based triple therapy for first-line Helicobacter pylori eradication. Aliment Pharmacol Ther. 2022;56(3):436–49.
Hu J, Mei H, Su NY, et al. Eradication rates of Helicobacter pylori in treatment-naive patients following 14-day vonoprazan-amoxicillin dual therapy: a multicenter randomized controlled trial in China. Helicobacter. 2023;28(4): e12970.
Hu Y, Xu X, Liu XS, et al. Fourteen-day vonoprazan and low- or high-dose amoxicillin dual therapy for eradicating Helicobacter pylori infection: a prospective, open-labeled, randomized non-inferiority clinical study. Front Immunol. 2022;13:1049908.
Yang F, Yu B, Qin L, et al. A randomized clinical study on the efficacy of vonoprazan combined with amoxicillin duo regimen for the eradication of Helicobacter pylori. Medicine (Baltimore). 2023;102(41): e35610.
Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther. 2017;46(2):106–14.
Shinozaki S, Kobayashi Y, Osawa H, et al. Effectiveness and safety of vonoprazan versus proton pump inhibitors for second-line Helicobacter pylori eradication therapy: systematic review and meta-analysis. Digestion. 2021;102(3):319–25.
Li M, Oshima T, Horikawa T, et al. Systematic review with meta-analysis: vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter. 2018;23(e12495):1–8.
Yang C, Li S, Huang T, et al. Effectiveness and safety of vonoprazan-based regimen for Helicobacter pylori eradication: a meta-analysis of randomized clinical trials. J Clin Pharm Ther. 2022;47(7):897–904.
Malfertheiner P, Moss SF, Daniele P, et al. Potassium-competitive acid blocker and proton pump inhibitor-based regimens for first-line Helicobacter pylori eradication: a network meta-analysis. Gastro Hep Adv. 2022;1(5):824–34.
Sun Y, Yue L, Hu W. Effectiveness and safety of vonoprazan-based regimens compared with those of proton pump inhibitor (PPI)-based regimens as first-line agents for Helicobacter pylori: a meta-analysis of randomized clinical trials. Eur J Clin Pharmacol. 2023;79(2):279–88.
Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67.
Fallone CA. The current role of vonoprazan in Helicobacter pylori treatment. Gastroenterology. 2022;163(3):572–4.
Acknowledgements
During the peer review process the manufacturer of Voquezna® Triple Pak and Voquezna® Dual Pak was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Matt Shirley is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: E. A. Argueta, Division of Gastroenterology, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, RI, USA; R. H. Hunt, Division of Gastroenterology and Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, Ontario, Canada.
The original online version of this article was revised due to retrospective open access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shirley, M. Vonoprazan: A Review in Helicobacter pylori Infection. Drugs 84, 319–327 (2024). https://doi.org/10.1007/s40265-023-01991-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01991-5