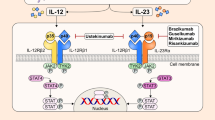
Abstract
Mirikizumab (Omvoh®), a humanized IgG4 anti-human IL-23p19 monoclonal antibody, is being developed by Eli Lilly and Company Ltd for the treatment of ulcerative colitis and Crohn’s disease. Mirikizumab was approved in March 2023 in Japan for use as induction and maintenance therapy in patients with moderate to severe ulcerative colitis who have an inadequate response to conventional therapy or therapies and is the first IL-23p19 inhibitor to be approved for this indication. Mirikizumab was granted a positive opinion in the EU in March 2023 for the treatment of adult patients with moderately to severely active UC who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a biologic treatment. This article summarizes the milestones in the development of mirikizumab leading to this first approval for use in ulcerative colitis.
Similar content being viewed by others
References
Almradi A, Hanzel J, Sedano R, et al. Clinical trials of IL-12/IL-23 inhibitors in inflammatory bowel disease. BioDrugs. 2020;34(6):713–21.
Nigam GB, Limdi JK. An update on the role of anti-IL-12/IL23 agents in the management of inflammatory bowel disease. Br Med Bull. 2021;138(1):29–40.
Noviello D, Mager R, Roda G, et al. The IL23-IL17 immune axis in the treatment of ulcerative colitis: successes, defeats, and ongoing challenges. Front Immunol. 2021;12: 611256.
Parigi TL, Iacucci M, Ghosh S. Blockade of IL-23: what is in the pipeline? J Crohns Colitis. 2022;16(Suppl 2):ii64–72.
Eli Lilly Japan KK. Omvoh® (mirikizumab): Japanese prescribing information. 2023. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/23994A5. Accessed 3 May 2023.
Eli Lilly Japan KK. Omvoh® (mirikizumab): information on acquisition of manufacturing and marketing approval. 2023. https://www.lillymedical.jp. Accessed 3 May 2023.
European Medicines Agency. Summary of opinion: Omvoh (mirikizumab). 2023. https://www.ema.europa.eu. Accessed 3 May 2023.
Eli Lilly and Company Ltd. U.S. Food and Drug Administration issues complete response letter for mirikizumab [media release]. 13 April 2023. https://investor.lilly.com.
Eli Lilly and Company Ltd. Lilly delivers first-quarter 2021 financial results, adjusts 2021 financial guidance [media release]. 27 April 2021. https://investor.lilly.com/.
Blauvelt A, Kimball AB, Augustin M, et al. Efficacy and safety of mirikizumab in psoriasis: results from a 52-week, double-blind, placebo-controlled, randomized withdrawal, phase III trial (OASIS-1). Br J Dermatol. 2022;187(6):866–77.
Papp K, Warren RB, Green LJ, et al. Efficacy and safety of mirikizumab versus secukinumab and placebo in the treatment of moderate-to-severe psoriasis: 52-week results from OASIS-2, a multicenter, randomized, double-blind study [abstract]. J Clin Aesthet Dermatol. 2021;14(5 Suppl 1):S26.
ClinicalTrials.gov. A long-term study to evaluate safety and maintenance of treatment effect of LY3074828 in participants with moderate-to-severe plaque psoriasis (OASIS-3). 2023. https://clinicaltrials.gov/ct2/show/results/NCT03556202. Accessed 3 May 2023.
Steere B, Schmitz J, Powell N, et al. Mirikizumab regulates genes involved in ulcerative colitis disease activity and anti-TNF resistance: results from a phase 2 study. Clin Transl Gastroenterol. 2023. https://doi.org/10.14309/ctg.0000000000000578.
Siegmund B, Sands BE, Samaan KH, et al. The effect of mirikizumab on fecal calprotectin and c-reactive protein in phase 3 studies of patients with moderately to severely active ulcerative colitis [abstract no. MP284]. United Eur Gastroenterol J. 2022;10(S8):384–5.
Panaccione R, Sapin C, Chan-Diehl FW, et al. The association of endoscopic and histologic endpoints with faecal calprotectin and C-reactive protein in patients with moderately to severely active ulcerative colitis treated with mirikizumab [abstract no. DOP90]. J Crohns Colitis. 2023;17(3):i167–8.
D’Haens G, Kobayashi T, Morris N, et al. Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-1 study [abstract no. 884]. Gastroenterology. 2022;162(7):S-214.
Dubinsky MC, Irving PM, Li X, et al. Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 study [abstract no. 867e]. Gastroenterology. 2022;162(7):S-1393-4.
Sands BE, Peyrin-Biroulet L, Kierkus J, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022;162(2):495–508.
Kaplan J., Bousvaros A., Turner D., et al. PK, efficacy and safety of mirikizumab as induction therapy in pediatric patients with moderately to severely active ulcerative colitis: results from the phase 2 SHINE-1 study [abstract no. 781]. In: Digestive Disease Week. 2023.
Magro F, Pai RK, Kobayashi T, et al. Resolving histologic inflammation in ulcerative colitis with mirikizumab in the LUCENT induction and maintenance trial programs. J Crohns Colitis. 2023. https://doi.org/10.1093/ecco-jcc/jjad050.
Sands BE, Feagan B, Gibble TH, et al. Mirikizumab improves quality of life in moderately-to-severely active UC: improvement in IBDQ scores in participants of LUCENT-1 and LUCENT-2 randomized, double-blind, placebo-controlled phase 3 trials [abstract no. A31]. J Can Assoc Gastroenterol. 2023;6(Suppl. 1):16–7.
D’Haens G, Higgins PDR, Peyrin-Biroulet L, et al. Extended induction response in patients treated with mirikizumab with moderately to severely active ulcerative colitis in the LUCENT trials [abstract no. P554]. J Crohns Colitis. 2023;17(Suppl. 1):i682–3.
Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology. 2020;158(3):537-49.e10.
Panaccione R, Feagan BG, Redondo I, et al. Efficacy and safety of mirikizumab in patients with ulcerative colitis: 104-week results from a phase 2 randomised controlled trial [abstract no. P0425]. United European Gastroenterol J. 2021;9(Suppl 8):516–7.
Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of continued treatment with mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20(1):105-15.e14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of Interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Susan J. Keam is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keam, S.J. Mirikizumab: First Approval. Drugs 83, 1045–1052 (2023). https://doi.org/10.1007/s40265-023-01909-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01909-1