Abstract
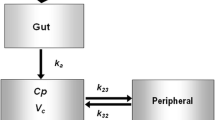
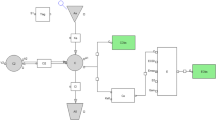
PB201 is an orally active, partial glucokinase activator targeting both pancreatic and hepatic glucokinase. As the second glucokinase activator studied beyond phase I, PB201 has demonstrated promising glycemic effects as well as favorable pharmacokinetic (PK) and safety profiles in patients with type 2 diabetes mellitus (T2DM). This study aims to develop a population PK/pharmacodynamic (PD) model for PB201 using the pooled data from nine phase I/II clinical trials conducted in non-Chinese healthy volunteers and a T2DM population and to predict the PK/PD profile of PB201 in a Chinese T2DM population. We developed the PK/PD model using the non-linear mixed-effects modeling approach. All runs were performed using the first-order conditional estimation method with interaction. The pharmacokinetics of PB201 were well fitted by a one-compartment model with saturable absorption and linear elimination. The PD effects of PB201 on reducing the fasting plasma glucose and glycosylated hemoglobin levels in the T2DM population were described by indirect response models as stimulating the elimination of fasting plasma glucose, where the production of glycosylated hemoglobin was assumed to be stimulated by fasting plasma glucose. Covariate analyses revealed enhanced absorption of PB201 by food and decreased systemic clearance with ketoconazole co-administration, while no significant covariate was identified for the pharmacodynamics. The population PK model established for non-Chinese populations was shown to be applicable to the Chinese T2DM population as verified by the PK data from the Chinese phase I study. The final population PK/PD model predicted persistent and dose-dependent reductions in fasting plasma glucose and glycosylated hemoglobin levels in the Chinese T2DM population receiving 50/50 mg, 100/50 mg, and 100/100 mg PB201 twice daily for 24 weeks independent of co-administration of metformin. Overall, the proposed population PK/PD model quantitatively characterized the PK/PD properties of PB201 and the impact of covariates on its target populations, which allows the leveraging of extensive data in non-Chinese populations with the limited data in the Chinese T2DM population to successfully supported the waiver of the clinical phase II trial and facilitate the optimal dose regimen design of a pivotal phase III study of PB201 in China.




Similar content being viewed by others
References
American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl. 1):S17–38.
American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl. 1):S125–43.
Ji LN, Lu JM, Guo XH, Yang WY, Weng JP, Jia WP, et al. Glycemic control among patients in China with type 2 diabetes mellitus receiving oral drugs or injectables. BMC Public Health. 2013;21(13):602.
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790.
Matschinsky FM, Wilson DF. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Front Physiol. 2019;10:148.
Doliba NM, Qin W, Najafi H, Liu C, Buettger CW, Sotiris J, et al. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab. 2012;302(1):E87-102.
Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005;5(3):171–6.
Ren Y, Li L, Wan L, Huang Y, Cao S. Glucokinase as an emerging anti-diabetes target and recent progress in the development of its agonists. J Enzyme Inhib Med Chem. 2022;37(1):606–15.
Spasov AA, Lobasenko VS, Kosolapov VA, Babkov DA, Kuznetsova VA, Maika OY, et al. Synthesis and pharmacological activity of 3-phenoxybenzoic acid derivatives. Pharm Chem J. 2020;54(3):229–35.
Dzyurkevich MS, Babkov DA, Shtyrlin NV, Mayka OY, Iksanova AG, Vassiliev PM, et al. Pyridoxine dipharmacophore derivatives as potent glucokinase activators for the treatment of type 2 diabetes mellitus. Sci Rep. 2017;7(1):16072.
Li W, Zhang X, Sun Y, Liu Z. Recent clinical advances of glucokinase activators in the treatment of diabetes mellitus type 2. Pharmazie. 2020;75(6):230–5.
Toulis KA, Nirantharakumar K, Pourzitaki C, Barnett AH, Tahrani AA. Glucokinase activators for type 2 diabetes: challenges and future developments. Drugs. 2020;80(5):467–75.
Zhi J, Zhai S. Effects of piragliatin, a glucokinase activator, on fasting and postprandial plasma glucose in patients with type 2 diabetes mellitus. J Clin Pharmacol. 2016;56(2):231–8.
Kiyosue A, Hayashi N, Komori H, Leonsson-Zachrisson M, Johnsson E. Dose-ranging study with the glucokinase activator AZD1656 as monotherapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(10):923–30.
Meininger GE, Scott R, Alba M, Shentu Y, Luo E, Amin H, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34(12):2560–6.
Yang W, Zhu D, Gan S, Dong X, Su J, Li W, et al. Dorzagliatin add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2022;28(5):974–81.
Pfefferkorn JA, Guzman-Perez A, Oates PJ, Litchfield J, Aspnes G, Basak A, et al. Designing glucokinase activators with reduced hypoglycemia risk: discovery of N, N-dimethyl-5-(2-methyl-6-((5-methylpyrazin-2-yl)-carbamoyl)benzofuran-4-yloxy)pyrimidine-2-carboxamide as a clinical candidate for the treatment of type 2 diabetes mellitus. Medchemcomm. 2011;2(9):828–39.
Amin NB, Aggarwal N, Pall D, Paragh G, Denney WS, Le V, et al. Two dose-ranging studies with PF-04937319, a systemic partial activator of glucokinase, as add-on therapy to metformin in adults with type 2 diabetes. Diabetes Obes Metab. 2015;17(8):751–9.
Denney WS, Denham DS, Riggs MR, Amin NB. Glycemic effect and safety of a systemic, partial glucokinase activator, PF-04937319, in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomized, crossover, active-controlled study. Clin Pharmacol Drug Dev. 2016;5(6):517–27.
Liu D, Du Y, Yao X, Wei Y, Zhu J, Cui C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the glucokinase activator PB-201 and its effects on the glucose excursion profile in drug-naïve Chinese patients with type 2 diabetes: a randomised controlled, crossover, single-centre phase 1 trial. EClinicalMedicine. 2021;42: 101185.
Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11(2):371–80.
Gao W, Jusko WJ. Modeling disease progression and rosiglitazone intervention in type 2 diabetic Goto-Kakizaki rats. J Pharmacol Exp Ther. 2012;341(3):617–25.
Borzilleri KA, Pfefferkorn JA, Guzman-Perez A, Liu SP, Qiu XY, Chrunyk BA, et al. Optimizing glucokinase activator binding kinetics to lower in vivo hypoglycemia risk. Medchemcomm. 2014;5(6):802–7.
Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, et al. Associations between features of glucose exposure and A1C: the A1C-Derived Average Glucose (ADAG) study. Diabetes. 2010;59(7):1585–90.
Gaitonde P, Garhyan P, Link C, Chien JY, Trame MN, Schmidt S. A comprehensive review of novel drug-disease models in diabetes drug development. Clin Pharmacokinet. 2016;55(7):769–88.
Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11(3):558–69.
Sharma R, Litchfield J, Atkinson K, Eng H, Amin NB, Denney WS, et al. Metabolites in safety testing assessment in early clinical development: a case study with a glucokinase activator. Drug Metab Dispos. 2014;42(11):1926–39.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The study was supported by the National Natural Science Foundation of China (no. 82173895 and no. 82373954), The Bill & Melinda Gates Foundation (Grant/Award Number: INV-007625) and the Peking University Third Hospital Clinical Project (Grant/Award Number: BYSYFY2021006).
Conflicts of interest/competing interests
Michael Xu, Ruifang Liang, Ke Ding, and Zhigang Lin are employees of PegBio Co., Ltd., (Suzhou, Jiangsu, China) the company that owns PB201. Ling Song, Fangrui Cao, and Dongyang Liu have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The data used in this study were collected in line with the principles of the Declaration of Helsinki. Approval was granted by institutional review boards and independent ethics committees for each study of which data were used in this work.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Authors’ contributions
LS, FC, and DL designed the study. LS and FC conducted the modeling and simulation work. MX, RL, KD, and ZL provided the clinical raw data.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, L., Cao, F., Niu, S. et al. Population Pharmacokinetic/Pharmacodynamic Analysis of the Glucokinase Activator PB201 in Healthy Volunteers and Patients with Type 2 Diabetes Mellitus: Facilitating the Clinical Development of PB201 in China. Clin Pharmacokinet 63, 93–108 (2024). https://doi.org/10.1007/s40262-023-01321-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01321-8