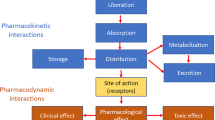
Abstract
Protein kinase inhibitors share pharmacokinetic (PK) pathways among themselves. They are all metabolized by several cytochromes P450 (CYP). For most of them, CYP3A4 is the predominant metabolic pathway. However, their oral bioavailability differs. For example, the oral bioavailability of imatinib has been estimated at nearly 100%, but that of ibrutinib averages 3% due to its high hepatic first-pass effect. Overall, the smaller the oral bioavailability, the larger its interindividual PK variability. Indeed, for drugs with low oral bioavailability, the extent of their absorption is an additional cause (along with elimination variability) of differences in drug exposure among patients. The impact of drug–drug interaction (DDI) also differs between drugs with low or high oral bioavailability. We describe and explain why the impact of CYP3A4 inhibitors and inducers is much greater for protein kinase inhibitors with low oral bioavailability. The effect of food on protein kinase inhibitors and DDIs corresponding to plasma protein binding will also be considered. Finally, the benefits of these concepts in clinical practice (including therapeutic drug monitoring) will be discussed. Overall, our main objective was to apply fundamental PK concepts to understanding the main clinical issues of these oral anticancer drugs.



Similar content being viewed by others
References
Pan Z, Scheerens H, Li S-J, Schultz BE, Sprengeler PA, Burrill LC, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2:58–61.
Ke E-E, Wu Y-L. EGFR as a pharmacological target in EGFR-mutant non-small-cell lung cancer: where do we stand now? Trends Pharmacol Sci. 2016;37:887–903.
Gougis P, Wassermann J, Spano JP, Keynan N, Funck-Brentano C, Salem JE. Clinical pharmacology of anti-angiogenic drugs in oncology. Crit Rev Oncol Hematol. 2017;119:75–93.
Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J Hematol Oncol. 2018;11:84.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502.
Carrato Mena A, Grande Pulido E, Guillén-Ponce C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs. 2010;21:S3.
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–23.
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. The Lancet. 2011;378:1931–9.
van der Graaf WT, Blay J-Y, Chawla SP, Kim D-W, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2012;379:1879–86.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–37.
Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37:1285–95.
Yang K, Fu L. Mechanisms of resistance to BCR–ABL TKIs and the therapeutic strategies: a review. Crit Rev Oncol Hematol. 2015;93:277–92.
Peng B, Dutreix C, Mehring G, Hayes MJ, Ben-Am M, Seiberling M, et al. Absolute bioavailability of imatinib (Glivec®) orally versus intravenous infusion. J Clin Pharmacol. 2004;44:158–62.
Center for Drug Evaluation and Research [CDER], US FDA. Clinical Pharmacology and Biopharmaceutics Review(s): ibrutinib. Silver Spring, MD; US FDA; 2013. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/205552orig1s000clinpharmr.pdf. (cited 2022 Oct 10)
Zhang W, McIntyre C, Forbes H, Gaafar R, Kohail H, Beck JT, et al. Effect of rifampicin on the pharmacokinetics of a single dose of vemurafenib in patients with BRAF V600 mutation-positive metastatic malignancy. Clin Pharmacol Drug Dev. 2019;8:837–43.
Tanaka C, Yin OQP, Smith T, Sethuraman V, Grouss K, Galitz L, et al. Effects of rifampin and ketoconazole on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol. 2011;51:75–83.
Dutreix C, Peng B, Mehring G, Hayes M, Capdeville R, Pokorny R, et al. Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol. 2004;54:290–4.
Jong J, Skee D, Murphy J, Sukbuntherng J, Hellemans P, Smit J, et al. Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol Res Perspect. 2015;3(4):e00156. https://doi.org/10.1002/prp2.156. (cited 2022 Sep 26).
Rowland M, Benet LZ, Graham GG. Clearance concepts in pharmacokinetics. J Pharmacokinet Biopharm. 1973;1:123–36.
Benet LZ. Clearance (née Rowland) concepts: a downdate and an update. J Pharmacokinet Pharmacodyn. 2010;37:529–39.
Pang KS, Han YR, Noh K, Lee PI, Rowland M. Hepatic clearance concepts and misconceptions: why the well-stirred model is still used even though it is not physiologic reality? Biochem Pharmacol. 2019;169: 113596.
Wilkinson GR, Shand DG. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18:377–90.
Tod M, Goutelle S, Bleyzac N, Bourguignon L. A generic model for quantitative prediction of interactions mediated by efflux transporters and cytochromes: application to P-glycoprotein and cytochrome 3A4. Clin Pharmacokinet. 2019;58:503–23.
Deng Y, Sychterz C, Suttle AB, Dar MM, Bershas D, Negash K, et al. Bioavailability, metabolism and disposition of oral pazopanib in patients with advanced cancer. Xenobiotica. 2013;43:443–53.
Tan AR, Gibbon DG, Stein MN, Lindquist D, Edenfield JW, Martin JC, et al. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1635–43.
Rakhit A, Pantze MP, Fettner S, Jones HM, Charoin J-E, Riek M, et al. The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: computer-based simulation (SimCYPTM) predicts in vivo metabolic inhibition. Eur J Clin Pharmacol. 2008;64:31–41.
Scheers E, Leclercq L, de Jong J, Bode N, Bockx M, Laenen A, et al. Absorption, metabolism, and excretion of oral 14C radiolabeled ibrutinib: an open-label, phase I, single-dose study in healthy men. Drug Metab Dispos. 2015;43:289–97.
Heath EI, Chiorean EG, Sweeney CJ, Hodge JP, Lager JJ, Forman K, et al. A phase I study of the pharmacokinetic and safety profiles of oral pazopanib with a high-fat or low-fat meal in patients with advanced solid tumors. Clin Pharmacol Ther. 2010;88:818–23.
Pavlović N, Goločorbin-Kon S, Ðanić M, Stanimirov B, Al-Salami H, Stankov K, et al. Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front Pharmacol. 2018;9:1283.
Lubberman FJE, Gelderblom H, Hamberg P, Vervenne WL, Mulder SF, Jansman FGA, et al. The effect of using pazopanib with food vs. fasted on pharmacokinetics, patient safety, and preference (DIET Study). Clin Pharmacol Ther. 2019;106(5):1076–82.
Devriese LA, Koch KM, Mergui-Roelvink M, Matthys GM, Ma WW, Robidoux A, et al. Effects of low-fat and high-fat meals on steady-state pharmacokinetics of lapatinib in patients with advanced solid tumours. Invest New Drugs. 2014;32:481–8.
Tsuda M, Ishiguro H, Toriguchi N, Masuda N, Bando H, Ohgami M, et al. Overnight fasting before lapatinib administration to breast cancer patients leads to reduced toxicity compared with nighttime dosing: a retrospective cohort study from a randomized clinical trial. Cancer Med. 2020;9:9246–55.
van Leeuwen RWF, Jansman FGA, Hunfeld NG, Peric R, Reyners AKL, Imholz ALT, et al. Tyrosine kinase inhibitors and proton pump inhibitors: an evaluation of treatment options. Clin Pharmacokinet. 2017;56:683–8.
van Leeuwen RWF, Peric R, Hussaarts KGAM, Kienhuis E, IJzerman NS, de Bruijn P, et al. Influence of the acidic beverage cola on the absorption of erlotinib in patients with non–small-cell lung cancer. J Clin Oncol. 2016;34:1309–14.
Knoebel RW, Larson RA. Pepsi® or Coke®? Influence of acid on dasatinib absorption. J Oncol Pharm Pract. 2018;24:156–8.
Mir O, Touati N, Lia M, Litière S, Le Cesne A, Sleijfer S, et al. Impact of concomitant administration of gastric acid-suppressive agents and pazopanib on outcomes in soft-tissue sarcoma patients treated within the EORTC 62043/62072 trials. Clin Cancer Res. 2019;25:1479–85.
Benet LZ, Hoener B-A. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71:115–21.
Berezhkovskiy LM. On the influence of protein binding on pharmacological activity of drugs. J Pharm Sci. 2010;99:2153–65.
Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92:203–13.
Hornecker M, Blanchet B, Billemont B, Sassi H, Ropert S, Taieb F, et al. Saturable absorption of sorafenib in patients with solid tumors: a population model. Invest New Drugs. 2012;30:1991–2000.
Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–7.
Groenland SL, van Eerden RAG, Verheijen RB, de Vries N, Thijssen B, Rosing H, et al. Cost-neutral optimization of pazopanib exposure by splitting intake moments: a prospective pharmacokinetic study in cancer patients. Clin Pharmacokinet. 2020;59:941–8.
Dallinger C, Trommeshauser D, Marzin K, Liesener A, Kaiser R, Stopfer P. Pharmacokinetic properties of nintedanib in healthy volunteers and patients with advanced cancer. J Clin Pharmacol. 2016;56:1387–94.
Swaisland HC, Smith RP, Laight A, Kerr DJ, Ranson M, Wilder-Smith CH, et al. Single-dose clinical pharmacokinetic studies of gefitinib. Clin Pharmacokinet. 2005;44:1165–77.
Swaisland HC, Ranson M, Smith RP, Leadbetter J, Laight A, McKillop D, et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet. 2005;44:1067–81.
Marzin K, Kretschmar G, Luedtke D, Kraemer S, Kuelzer R, Schlenker-Herceg R, et al. Pharmacokinetics of nintedanib in subjects with hepatic impairment. J Clin Pharmacol. 2018;58:357–63.
Westerdijk K, Desar IME, Steeghs N, van der Graaf WTA, van Erp NP, on behalf of the Dutch Pharmacology and Oncology Group (DPOG). Imatinib, sunitinib and pazopanib: from flat-fixed dosing towards a pharmacokinetically guided personalized dose. Br J Clin Pharmacol. 2020;86:258–73.
Cerbone L, Combarel D, Geraud A, Auclin E, Foulon S, Alve Costa Silva C, et al. Association of cabozantinib pharmacokinetics, progression and toxicity in metastatic renal cell carcinoma patients: results from a pharmacokinetics/pharmacodynamics study. ESMO Open. 2021;6:100312.
Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR. Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin Pharmacol Ther. 2017;102:765–76.
Le Louedec F, Puisset F, Thomas F, Chatelut É, White-Koning M. Easy and reliable maximum a posteriori Bayesian estimation of pharmacokinetic parameters with the open-source R package mapbayr. CPT Pharmacomet Syst Pharmacol. 2021;10(10):1208–20.
Corral Alaejos Á, Zarzuelo Castañeda A, Jiménez Cabrera S, Sánchez-Guijo F, Otero MJ, Pérez-Blanco JS. External evaluation of population pharmacokinetic models of imatinib in adults diagnosed with chronic myeloid leukaemia. Br J Clin Pharmacol. 2022;88(4):1913–24. https://doi.org/10.1111/bcp.15122.
Lacy S, Yang B, Nielsen J, Miles D, Nguyen L, Hutmacher M. A population pharmacokinetic model of cabozantinib in healthy volunteers and patients with various cancer types. Cancer Chemother Pharmacol. 2018;81:1071–82.
Delbaldo C, Chatelut E, Ré M, Deroussent A, Séronie-Vivien S, Jambu A, et al. Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res. 2006;12:6073–8.
Marostica E, Sukbuntherng J, Loury D, de Jong J, de Trixhe XW, Vermeulen A, et al. Population pharmacokinetic model of ibrutinib, a Bruton tyrosine kinase inhibitor, in patients with B cell malignancies. Cancer Chemother Pharmacol. 2015;75:111–21.
Duan JZ, Jackson AJ, Zhao P. Bioavailability considerations in evaluating drug-drug interactions using the population pharmacokinetic approach. J Clin Pharmacol. 2011;51:1087–100.
Bolton AE, Peng B, Hubert M, Krebs-Brown A, Capdeville R, Keller U, et al. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol. 2004;53:102–6.
US Food and Drug Administration. FDA; 2022 [cited 2022 Nov 16]. https://www.fda.gov/home.
PubChem [cited 2022 Nov 16]. https://pubchem.ncbi.nlm.nih.gov/.
Podoll T, Pearson PG, Evarts J, Ingallinera T, Bibikova E, Sun H, et al. Bioavailability, biotransformation, and excretion of the covalent bruton tyrosine kinase inhibitor acalabrutinib in rats, dogs, and humans. Drug Metab Dispos. 2019;47:145–54.
Center for Drug Evaluation and Research [CDER], US FDA. Multi-Discipline Review: acalabrutinib. Silver Spring, MD: US FDA; 2017 [cited 2022 Sep 26]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/210259Orig1s000MultidisciplineR.pdf.
Wind S, Giessmann T, Jungnik A, Brand T, Marzin K, Bertulis J, et al. Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clin Drug Investig. 2014;34:173–82.
Center for Drug Evaluation and Research [CDER], US FDA. Clinical Pharmacology and Biopharmaceutics Review(s): afatinib. Silver Spring, MD: US FDA; 2012 [cited 2022 Sep 26]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/201292Orig1s000ClinPharmR.pdf.
Morcos PN, Yu L, Bogman K, Sato M, Katsuki H, Kawashima K, et al. Absorption, distribution, metabolism and excretion (ADME) of the ALK inhibitor alectinib: results from an absolute bioavailability and mass balance study in healthy subjects. Xenobiotica. 2017;47:217–29.
Morcos PN, Cleary Y, Guerini E, Dall G, Bogman K, De Petris L, et al. Clinical drug-drug interactions through cytochrome P450 3A (CYP3A) for the selective ALK inhibitor alectinib. Clin Pharmacol Drug Dev. 2017;6:280–91.
European Medicines Agency. Inlyta: EPAR-Product Information. Annex I-Summary of product characteristics. Amsterdam: European Medicines Agency; 2012 [cited 2019 Jul 23]. https://www.ema.europa.eu/en/documents/product-information/inlyta-epar-product-information_en.pdf.
Pithavala YK, Tong W, Mount J, Rahavendran SV, Garrett M, Hee B, et al. Effect of ketoconazole on the pharmacokinetics of axitinib in healthy volunteers. Invest New Drugs. 2012;30:273–81.
Pithavala YK, Tortorici M, Toh M, Garrett M, Hee B, Kuruganti U, et al. Effect of rifampin on the pharmacokinetics of Axitinib (AG-013736) in Japanese and Caucasian healthy volunteers. Cancer Chemother Pharmacol. 2010;65:563–70.
Abbas R, Boni J, Sonnichsen D. Effect of rifampin on the pharmacokinetics of bosutinib, a dual Src/Abl tyrosine kinase inhibitor, when administered concomitantly to healthy subjects. Drug Metab Person Ther. 2015;30(1):57–63.
Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D. Effect of ketoconazole on the pharmacokinetics of oral bosutinib in healthy subjects. J Clin Pharmacol. 2011;51:1721–7.
Hsyu P-H, Pignataro DS, Matschke K. Absolute bioavailability of bosutinib in healthy subjects from an open-label, randomized, 2-period crossover study. Clin Pharmacol Drug Dev. 2018;7:373–81.
Center for Drug Evaluation and Research [CDER], US FDA. Clinical Pharmacology and Biopharmaceutics Review(s): ceritinib. Silver Spring, MD: US FDA; 2014 [cited 2022 Sep 26]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205755orig1s000clinpharmr.pdf.
Xu H, O’Gorman M, Boutros T, Brega N, Kantaridis C, Tan W, et al. Evaluation of crizotinib absolute bioavailability, the bioequivalence of three oral formulations, and the effect of food on crizotinib pharmacokinetics in healthy subjects. J Clin Pharmacol. 2015;55:104–13.
Xu H, O’Gorman M, Tan W, Brega N, Bello A. The effects of ketoconazole and rifampin on the single-dose pharmacokinetics of crizotinib in healthy subjects. Eur J Clin Pharmacol. 2015;71:1441–9.
European Medicines Agency. Tafinlar: EPAR-Product Information. Annex I-Summary of product characteristics. Amsterdam: European Medicines Agency; 2018 [cited 2022 Nov 16]. https://www.ema.europa.eu/en/documents/product-information/tafinlar-epar-product-information_en.pdf.
Meneses-Lorente G, Bentley D, Guerini E, Kowalski K, Chow-Maneval E, Yu L, et al. Characterization of the pharmacokinetics of entrectinib and its active M5 metabolite in healthy volunteers and patients with solid tumors. Invest New Drugs. 2021;39:803–11.
Meneses-Lorente G, Fowler S, Guerini E, Kowalski K, Chow-Maneval E, Yu L, et al. In vitro and clinical investigations to determine the drug-drug interaction potential of entrectinib, a small molecule inhibitor of neurotrophic tyrosine receptor kinase (NTRK). Invest New Drugs. 2022;40:68–80.
Frohna P, Lu J, Eppler S, Hamilton M, Wolf J, Rakhit A, et al. Evaluation of the ABSOLUTE ORAL BIOAVAILABILITY AND BIOEQUIVALENCE OF ERLOTINIB, AN INHIBITOR OF THE EPIDERMAL GROWTH FACTOR RECEPTOR TYROSINE KINASE, IN A RANDOMIZED, CROSSOVER STUDY IN HEALTHY SUBJECTS. J Clin Pharmacol. 2006;46:282–90.
Hamilton M, Wolf JL, Drolet DW, Fettner SH, Rakhit AK, Witt K, et al. The effect of rifampicin, a prototypical CYP3A4 inducer, on erlotinib pharmacokinetics in healthy subjects. Cancer Chemother Pharmacol. 2014;73:613–21.
Hibma JE, O’Gorman M, Nepal S, Pawlak S, Ginman K, Pithavala YK. Evaluation of the absolute oral bioavailability of the anaplastic lymphoma kinase/c-ROS oncogene 1 kinase inhibitor lorlatinib in healthy participants. Cancer Chemother Pharmacol. 2022;89:71–81.
Patel M, Chen J, McGrory S, O’Gorman M, Nepal S, Ginman K, et al. The effect of itraconazole on the pharmacokinetics of lorlatinib: results of a phase I, open-label, crossover study in healthy participants. Invest New Drugs. 2020;38:131–9.
Chen J, Xu H, Pawlak S, James LP, Peltz G, Lee K, et al. The Effect of rifampin on the pharmacokinetics and safety of lorlatinib: results of a phase one, open-label, crossover study in healthy participants. Adv Ther. 2020;37:745–58.
Center for Drug Evaluation and Research [CDER], US FDA. Multi-Discipline Review: mobocertinib [Internet]. Silver Spring, MD: US FDA; 2021 [cited 2022 Nov 16]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215310Orig1s000MultidisciplineR.pdf.
European Medicines Agency. Tasigna: EPAR - Product Information. Annex I - Summary of product characteristics. Amsterdam: European Medicines Agency; 2007 [cited 2022 Sep 26]. https://ec.europa.eu/health/documents/community-register/2022/20220328155427/anx_155427_en.pdf.
Vishwanathan K, So K, Thomas K, Bramley A, English S, Collier J. Absolute bioavailability of osimertinib in healthy adults. Clin Pharmacol Drug Dev. 2019;8:198–207.
Vishwanathan K, Dickinson PA, So K, Thomas K, Chen Y-M, De Castro CJ, et al. The effect of itraconazole and rifampicin on the pharmacokinetics of osimertinib: CYP3A effects on osimertinib PK. Br J Clin Pharmacol. 2018;84:1156–69.
Center for Drug Evaluation and Research [CDER], US FDA. Clinical Pharmacology and Biopharmaceutics Review(s): palbociclib. Silver Spring, MD: US FDA; 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207103orig1s000clinpharmr.pdf. (cited 2022 Oct 10)
Center for Drug Evaluation and Research [CDER], US FDA. Clinical Pharmacology and Biopharmaceutics Review(s): ponatinib. Silver Spring: US FDA; 2012.
Narasimhan NI, Dorer DJ, Niland K, Haluska F, Sonnichsen D. Effects of ketoconazole on the pharmacokinetics of ponatinib in healthy subjects. J Clin Pharmacol. 2013;53:974–81.
Narasimhan NI, Dorer DJ, Davis J, Turner CD, Sonnichsen D. Evaluation of the effect of multiple doses of rifampin on the pharmacokinetics and safety of ponatinib in healthy subjects. Clin Pharmacol Drug Dev. 2015;4:354–60.
Shi JG, Chen X, Emm T, Scherle PA, McGee RF, Lo Y, et al. The Effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 Phosphate) in healthy volunteers. J Clin Pharmacol. 2012;52:809–18.
Center for Drug Evaluation and Research [CDER], US FDA. Clinical Pharmacology and Biopharmaceutics Review(s): ruxolitinib. Silver Spring, MD: US FDA; 2011 [cited 2022 Sep 26]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202192Orig1s000ClinPharmR.pdf.
Center for Drug Evaluation and Research [CDER], US FDA. Multi-Discipline Review: selumetinib. Silver Spring, MD: US FDA; 2019 [cited 2022 Nov 16]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/213756Orig1s000MultidisciplineR.pdf.
Zhang W, Colburn D, Simmons B, Papai Z, Bertran E, Schadt S, et al. Absolute bioavailability of vemurafenib in patients with BRAFV600 mutation-positive malignancies. Clin Pharmacol Drug Dev. 2020;9:496–504.
Zhang W, Mathisen M, Goodman GR, Forbes H, Song Y, Bertran E, et al. Effect of itraconazole, a potent CYP3A4 inhibitor, on the steady-state pharmacokinetics of vemurafenib in patients with BRAFV600 mutation-positive malignancies. Clin Pharmacol Drug Dev. 2021;10:39–45.
Acknowledgments
The authors would like to thank Dr. Gail Taillefer, native English speaker experienced in scientific publication, for her review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were received to assist in the preparation of this article.
Conflicts of interest/competing interests
Félicien Le Louedec, Florent Puisset, Etienne Chatelut, and Michel Tod declare that they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have approved and share full responsibility for the manuscript, and give consent for publication.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Each author contributed to the writing and editing of this manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le Louedec, F., Puisset, F., Chatelut, E. et al. Considering the Oral Bioavailability of Protein Kinase Inhibitors: Essential in Assessing the Extent of Drug–Drug Interaction and Improving Clinical Practice. Clin Pharmacokinet 62, 55–66 (2023). https://doi.org/10.1007/s40262-022-01200-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01200-8