Abstract
Background
Tranexamic acid (TXA), an antifibrinolytic drug, is usually administered intravenously; however, intra-articular administration has recently been proven to be as effective as intravenous administration. Limited information regarding the pharmacokinetics (PK) of TXA after intra-articular administration has been reported.
Aims
The aim of this study was to develop a population PK model of TXA administered as a single intra-articular dose and as two intravenous doses, and to study the sources of interindividual variability (IIV) in the PK processes of TXA. The developed model was used to simulate PK profiles of TXA at different dosage regimens and in patients with renal impairment.
Methods
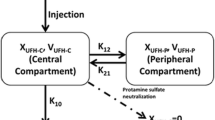
Patients who underwent primary unilateral total knee replacement (TKR) received 1 g/10 mL (concentration of 100 mg/mL) of TXA applied directly to the surgical field before wound closure, or 2 g (two doses of 1 g) of intravenous TXA. A population PK model was developed using a nonlinear mixed-effects approach and sources of IIV, such as sex, age, body weight, height, body mass index (BMI), preoperative haemoglobin, preoperative haematocrit, and creatinine clearance.
Results
Twenty-four patients were included, 12 in each group. Twenty patients were female, mean age (standard deviation) was 73.7 years (5.6). The disposition of TXA was best described as a two-compartment model with clearance dependent on creatinine clearance. Bootstrap results indicated that the model was stable and robust. The estimated bioavailability for intra-articular administration was 81%. Simulations indicated that 100% of patients would have plasma concentrations associated with partial fibrinolysis at 8 h post-administration with the dosages and routes of administration used in the present study. Intra-articular administration would produce complete inhibition of fibrinolysis in only 12% of patients compared with 72.5% with intravenous administration. No adverse events were reported.
Conclusions
This population PK model demonstrated that a single dose of high-concentration, low-volume intra-articular TXA can achieve antifibrinolytic plasma concentrations of the drug for 8 h, providing both local and systemic effects in patients undergoing TKR. TXA administration to the surgical field could be an alternative to the intravenous; route for patients undergoing TKR; however, clinical studies are needed to assess the toxic local effects of TXA.
Trial Registration
Spanish Clinical Studies Registry Number: 2017-004059-22. Date of registration: 12 April 2018.



Similar content being viewed by others
References
White CC 4th, Eichinger JK, Friedman RJ. Minimizing blood loss and transfusions in total knee arthroplasty. J Knee Surg. 2018;31(7):594–9. https://doi.org/10.1055/s-0038-1648223.
Aguilera X, Martínez-Zapata MJ, Hinarejos P, Jordán M, Leal J, González JC, et al. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg. 2015;135:1017–25.
CRASH-2 Trial Collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. https://doi.org/10.1016/S0140-6736(10)60835-5.
Picetti R, Shakur-Still H, Medcalf RL, Standing JF, Roberts I. What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul Fibrinolysis. 2019;30(1):1–10. https://doi.org/10.1097/MBC.0000000000000789.
Rong GX, Shen CL, Gui BJ, Yin H, Tang Z. Comparison of tranexamic acid pharmacokinetics after intra-articular and intravenous administration in rabbits. Pak J Pharm Sci. 2017;30(4):1309–16.
Sa-ngasoongsong P, Chanplakorn P, Wongsak S, Uthadorn K, Panpikoon T, Jittorntam P, et al. An in vivo study of low-dose intra-articular tranexamic acid application with prolonged clamping drain method in total knee replacement: clinical efficacy and safety. Biomed Res Int. 2015. https://doi.org/10.1155/2015/164206.
Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Jt Surg Am. 2010;92:2503–13. https://doi.org/10.2106/JBJS.I.01518.
Eriksson O, Kjellman H, Pilbrant A, Schannong M. Pharmacokinetics of tranexamic acid after intravenous administration to normal volunteers. Eur J Clin Pharmacol. 1974;7(5):375–80. https://doi.org/10.1007/BF00558210.
Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20(1):65–72. https://doi.org/10.1007/BF00554669.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26. https://doi.org/10.1007/s10928-007-9066-0.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. https://doi.org/10.1159/000180580.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM [published correction appears in Comput Methods Programs Biomed. 2005 Dec;80(3):277. Jonsson, Niclas (corrected to Jonsson, E Niclas)]. Comput Methods Programs Biomed. 2005;79(3):241–57. https://doi.org/10.1016/j.cmpb.2005.04.005.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51. https://doi.org/10.1208/s12248-011-9255-z.
Ausen K, Pleym H, Liu J, Hegstad S, Nordgård HB, Pavlovic I, et al. Serum concentrations and pharmacokinetics of tranexamic acid after two means of topical administration in massive weight loss skin-reducing surgery. Plast Reconstr Surg. 2019;143(6):1169e–78e. https://doi.org/10.1097/PRS.0000000000005620.
Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–32.
Jules-Elysee KM, Tseng A, Sculco TP, Baaklini LR, McLawhorn AS, Pickard AJ, et al. Comparison of topical and intravenous tranexamic acid for total knee replacement: a randomized double-blinded controlled study of effects on tranexamic acid levels and thrombogenic and inflammatory marker levels. J Bone Jt Surg Am. 2019;101(23):2120–8. https://doi.org/10.2106/JBJS.19.00258.
Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, et al. Measured and estimated GFR in healthy potential kidney donors [published erratum appears in Am J Kidney Dis. 2004 Dec;44(6):1126; and Am J Kidney Dis. 2005 Jul;46(1):170]. Am J Kidney Dis. 2004;43(1):112–9. https://doi.org/10.1053/j.ajkd.2003.09.026.
Tranexamic acid. Summary of product characteristics. https://www.medicines.org.uk/emc/product/1220/smpc#gref. Accessed 30 Nov 2020.
Mezzano D, Panes O, Muñoz B, Pais E, Tagle R, González F, et al. Tranexamic acid inhibits fibrinolysis, shortens the bleeding time and improves platelet function in patients with chronic renal failure. Thromb Haemost. 1999;82(4):1250–4 (PMID: 10544908).
Dowd NP, Karski JM, Cheng DC, Carroll JA, Lin Y, James RL, et al. Pharmacokinetics of tranexamic acid during cardiopulmonary bypass. Anesthesiology. 2002;97(2):390–9. https://doi.org/10.1097/00000542-200208000-00016.
Grassin-Delyle S, Tremey B, Abe E, Fischler M, Alvarez JC, Devillier P, et al. Population PKs of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2013;111(6):916–24. https://doi.org/10.1093/bja/aet255.
Drug Bank; June 13, 2005 [updated 5 Aug 2019]. https://www.drugbank.ca/drugs/DB00302. Accessed 8 Aug 2019.
Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery [published erratum appears in N Engl J Med. 2010 Sep 23;363(13):1290]. N Engl J Med. 2008;358:2319–31. https://doi.org/10.1056/NEJMoa0802395.
Karski JM, Teasdale SJ, Norman PH, Carroll JA, Weisel RD, Glynn MF. Prevention of post bypass bleeding with tranexamic acid and epsilon-aminocaproic acid. J Cardiothorac Vasc Anesth. 1993;7:431–5. https://doi.org/10.1016/1053-0770(93)90165-h.
Ngaage DL, Bland JM. Lessons from aprotinin: Is the routine use and inconsistent dosing of tranexamic acid prudent? Meta-analysis of randomised and large matched observational studies. Eur J Cardiothorac Surg. 2010;37:1375–83. https://doi.org/10.1016/j.ejcts.2009.11.055.
Li J, Liu R, Rai S, Ze R, Tang X, Hong P. Intra-articular vs intravenous administration: a meta-analysis of tranexamic acid in primary total knee arthroplasty. J Orthop Surg Res. 2020;15(1):581. https://doi.org/10.1186/s13018-020-02119-1.
Jiang T, Song K, Yao Y, Pan P, Jiang Q. Perioperative allogenic blood transfusion increases the incidence of postoperative deep vein thrombosis in total knee and hip arthroplasty. J Orthop Surg Res. 2019;14(1):235. https://doi.org/10.1186/s13018-019-1270-2.
Chornenki NLJ, Um KJ, Mendoza PA, et al. Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: a systematic review and meta-analysis. Thromb Res. 2019;179:81–6. https://doi.org/10.1016/j.thromres.2019.05.003.
Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: causes and treatment. Ann Neurol. 2016;79(1):18–26. https://doi.org/10.1002/ana.24558.
Bolam SM, O’Regan-Brown A, Paul Monk A, Musson DS, Cornish J, Munro JT. Toxicity of tranexamic acid (TXA) to intra-articular tissue in orthopaedic surgery: a scoping review. Knee Surg Sports Traumatol Arthrosc. 2021;29(6):1862–71. https://doi.org/10.1007/s00167-020-06219-7.
Acknowledgements
The authors thank the patients who participated in this study and the Research Institute of Hospital de la Santa Creu i Sant Pau—UICEC Sant Pau, which monitored the study. They also thank Jane Marshall for editing the manuscript. Aránzazu Gonzalez-Osuna is a PhD candidate in Surgery and Morphologic Sciences at the Autonomous University of Barcelona (Spain). Dr Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CPII20/00023). The members of FARMATX Study Group: Dr. Adriá Font Gual, Dr. Aránzazu González Osuna, Dr. Claudia Lamas, Dr. Eduard Ramirez, Mrs Esther Cánovas Martínez, Mr Francesc Pla-Junca, Dr. José Antonio Fernández Nuñez, Dr. José Carlos González Rodriguez, Ms Luisa Fernanda Rojas, Dr. Marcos Jordán Sales, Dr. Mireia Rodríguez Prieto, Dr. Mª José Martínez-Zapata, Dr. Marta Valle, Dr. Sebastián Videla, Dr. Victoria Baños Lapuente, Dr. Xavier Aguilera Roig.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
Aránzazu González Osuna, Luisa Fernanda Rojas, Claudia Lamas, Xavier Aguilera Roig, Francesc Pla-Junca, Sebastián Videla, Mª José Martínez-Zapata, and Marta Valle declare they have no conflicts of interest.
Funding
This study was supported by internal sources from the Institut de Recerca Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Ethics approval
The clinical trial was approved by the Investigational Review Board of Hospital de Santa Creu i Sant Pau, Barcelona, Spain. This study was conducted in accordance with the principles of the Declaration of Helsinki. A high level of confidentiality, in terms of protection of personal data as required by Spanish Law (LOPD 3/2018), was also ensured.
Consent to participate
Patients who agreed to participate signed a written informed consent form.
Consent for publication
Not applicable.
Availability of data and material
Research data are not shared.
Code availability
Not applicable. The clinical trial protocol was registered in the Spanish Registry of Clinical Studies database (https://reec.aemps.es; number 2017-004159-22).
Author contributions
AGO, MV, LFR, XAR, FP-J, MJM-Z, and SV were involved in the conception and design of the study and/or the analysis and interpretation of the data. AGO and XAR were involved in the acquisition of data. MV, LFR and FP-J performed the pharmacokinetics analysis. AGO, MV, LFR, XAR, MJM-Z, and SV wrote the manuscript. All authors revised the article critically for important intellectual content and gave approval for the final version to be published.
Additional information
The members of FARMATX Study Group are listed in acknowledgements.
Rights and permissions
About this article
Cite this article
González Osuna, A., Rojas, L.F., Lamas, C. et al. Population Pharmacokinetics of Intra-articular and Intravenous Administration of Tranexamic Acid in Patients Undergoing Total Knee Replacement. Clin Pharmacokinet 61, 83–95 (2022). https://doi.org/10.1007/s40262-021-01043-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01043-9