Abstract
Background and Objective
PSD502 is a metered-dose spray for premature ejaculation. The two trials aimed to evaluate the safety and pharmacokinetics of PSD502 in healthy Chinese male and female individuals.
Methods
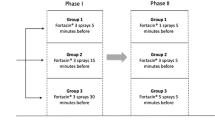
Two phase I, randomized, double-blind, placebo-controlled trials were conducted in men (Trial 1) and women (Trial 2). The participants were randomized 3:1 to receive PSD502 (7.5 mg of lidocaine and 2.5 mg of prilocaine per spray) or a placebo. For male individuals, a single dose (three sprays) once daily was applied to the glans penis for 21 days except for nine sprays (three doses) on days 7 and 14, 4 h apart for each dose. For female individuals, two sprays were applied to the vagina and one to the cervix once daily for 7 days. The primary endpoint was safety. Pharmacokinetics analysis was also performed.
Results
Twenty-four male and 24 female individuals were recruited. Treatment-emergent adverse events occurred in 38.9% (7/18) of male individuals and 66.7% (12/18) of female individuals in the PSD502 group, respectively. Both trials reported 50.0% (3/6) treatment-emergent adverse events for the placebo. No grade ≥ 3 treatment-emergent adverse events, serious adverse events, or treatment-emergent adverse events leading to early withdrawal or discontinuation occurred. After consecutive applications, lidocaine and prilocaine cleared rapidly in both trials. Plasma concentrations exhibited high inter-individual variability. The maximum plasma concentrations of active ingredients were far below the anticipated minimum toxic concentrations. The area under the plasma concentration–time curve of metabolites were ≤ 20% of the parent drugs. No clinically significant accumulations were observed in the two trials.
Conclusions
PSD502 was well tolerated and showed low plasma concentrations in healthy Chinese male and female individuals.



Similar content being viewed by others
References
Althof SE, McMahon CG, Waldinger MD, Serefoglu EC, Shindel AW, Adaikan PG, et al. An update of the International Society of Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation (PE). J Sex Med. 2014;11(6):1392–422.
Shindel AW, Althof SE, Carrier S, Chou R, McMahon CG, Mulhall JP, et al. Disorders of ejaculation: an AUA/SMSNA guideline. J Urol. 2022;207(3):504–12.
Porst H, Montorsi F, Rosen RC, Gaynor L, Grupe S, Alexander J. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol. 2007;51(3):816–23 (discussion 24).
Carson C, Gunn K. Premature ejaculation: definition and prevalence. Int J Impot Res. 2006;18(Suppl 1):S5-13.
Gao J, Zhang X, Su P, Liu J, Xia L, Yang J, et al. Prevalence and factors associated with the complaint of premature ejaculation and the four premature ejaculation syndromes: a large observational study in China. J Sex Med. 2013;10(7):1874–81.
Salonia A, Bettocchi C, Boeri L, Capogrosso P, Carvalho J, Cilesiz NC, et al. European Association of Urology guidelines on sexual and reproductive health-2021 update: male sexual dysfunction. Eur Urol. 2021;80(3):333–57.
Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, et al. AUA guideline on the pharmacologic management of premature ejaculation. J Urol. 2004;172(1):290–4.
Hellstrom WJ. Update on treatments for premature ejaculation. Int J Clin Pract. 2011;65(1):16–26.
Keel CE, Dorsey PJ, Acker W, Hellstrom WJ. New concepts in the diagnosis and treatment of premature ejaculation. Curr Urol Rep. 2010;11(6):414–20.
Hiemke C, Hartter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85(1):11–28.
Giuliano F, Clément P. Serotonin and premature ejaculation: from physiology to patient management. Eur Urol. 2006;50(3):454–66.
Mirone V, Arcaniolo D, Rivas D, Bull S, Aquilina JW, Verze P. Results from a prospective observational study of men with premature ejaculation treated with dapoxetine or alternative care: the PAUSE study. Eur Urol. 2014;65(4):733–9.
Wyllie MG, Hellstrom WJ. The link between penile hypersensitivity and premature ejaculation. BJU Int. 2011;107(3):452–7.
Atikeler MK, Gecit I, Senol FA. Optimum usage of prilocaine-lidocaine cream in premature ejaculation. Andrologia. 2002;34(6):356–9.
Busato W, Galindo CC. Topical anaesthetic use for treating premature ejaculation: a double-blind, randomized, placebo-controlled study. BJU Int. 2004;93(7):1018–21.
Martyn-St James M, Cooper K, Ren K, Kaltenthaler E, Dickinson K, Cantrell A, et al. Topical anaesthetics for premature ejaculation: a systematic review and meta-analysis. Sex Health. 2016;13(2):114–23.
Henry R, Morales A, Wyllie MG. TEMPE: topical eutectic-like mixture for premature ejaculation. Expert Opin Drug Deliv. 2008;5(2):251–61.
Dinsmore WW, Hackett G, Goldmeier D, Waldinger M, Dean J, Wright P, et al. Topical eutectic mixture for premature ejaculation (TEMPE): a novel aerosol-delivery form of lidocaine-prilocaine for treating premature ejaculation. BJU Int. 2007;99(2):369–75.
Carson C, Wyllie M. Improved ejaculatory latency, control and sexual satisfaction when PSD502 is applied topically in men with premature ejaculation: results of a phase III, double-blind, placebo-controlled study. J Sex Med. 2010;7(9):3179–89.
Dinsmore WW, Wyllie MG. PSD502 improves ejaculatory latency, control and sexual satisfaction when applied topically 5 min before intercourse in men with premature ejaculation: results of a phase III, multicentre, double-blind, placebo-controlled study. BJU Int. 2009;103(7):940–9.
Zhou Q, Yu X, Shu C, Cai Y, Gong W, Wang X, et al. Analysis of CYP3A4 genetic polymorphisms in Han Chinese. J Hum Genet. 2011;56(6):415–22.
Olafuyi O, Parekh N, Wright J, Koenig J. Inter-ethnic differences in pharmacokinetics: is there more that unites than divides? Pharmacol Res Perspect. 2021;9(6): e00890.
Cold CJ, Taylor JR. The prepuce. BJU Int. 1999;83(Suppl. 1):34–44.
Immerman RS, Mackey WC. A biocultural analysis of circumcision. Soc Biol. 1997;44(3–4):265–75.
McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20(11):1491–5.
Hellstrom W, Carson C, Wyllie M. 1494 PSD 502 appears to be effective in both circumcised and un-circumcised men with premature ejaculation (PE). J Urology. 2010;183(4S):e576-e.
McMahon CG. Dapoxetine: a new option in the medical management of premature ejaculation. Ther Adv Urol. 2012;4(5):233–51.
McMahon CG. Efficacy of dapoxetine in the treatment of premature ejaculation. Clin Med Insights Reprod Health. 2011;2(5):25–39.
Peng J, Fang D, Li H, Tang Y, Yuan Y, Cui W, et al. Efficacy of dapoxetine treatment in Chinese patients with premature ejaculation and possible factors affecting efficacy in the real-world practice. BMC Urol. 2020;20(1):11.
Buvat J, Tesfaye F, Rothman M, Rivas DA, Giuliano F. Dapoxetine for the treatment of premature ejaculation: results from a randomized, double-blind, placebo-controlled phase 3 trial in 22 countries. Eur Urol. 2009;55(4):957–67.
Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry. 2001;62(Suppl. 3):10–21.
McMahon C, Kim SW, Park NC, Chang CP, Rivas D, Tesfaye F, et al. Treatment of premature ejaculation in the Asia-Pacific region: results from a phase III double-blind, parallel-group study of dapoxetine. J Sex Med. 2010;7(1 Pt 1):256–68.
EMC. EMLA cream 5% (5g pack). 2017. https://www.medicines.org.uk/emc/product/871/smpc#gref. Accessed 18 May 2023.
Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–57.
Abernethy DR, Greenblatt DJ. Impairment of lidocaine clearance in elderly male subjects. J Cardiovasc Pharmacol. 1983;5(6):1093–6.
Acknowledgments
The authors acknowledge all the participants in these two trials and thank Xin Wang from Fosun Pharma for her contribution to the study design. Medical writing assistance was provided by MedSci and funded by Fosun Pharma.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
These studies were funded by Wanbang Biopharmaceuticals.
Conflict of interest
Lu Liu, Ziyang Liu, Yunan Sun, Lei Diao, and Jun Lu are full-time employees of Fosun Pharma. Yongchun Zhou is a full-time employee of Wanbang Biopharmaceuticals. Fangfang Wang, Zhiping Liu, Xiaoye Niu, Lin Zhao, Jixiang Zhu, Linjing Qi, Xiaoye Wang, and Haiyan Li have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The protocols of two trials (CTR20213292 and CTR20213291) were approved by the Institutional Review Board of Peking University Third Hospital (Beijing, China). The studies were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments and all Chinese regulatory requirements.
Consent to participate
Written informed consent was obtained from all subjects for these two studies before the screening.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Author contributions
Conceptualization: LD, JL, YZ, XW, and HL; methodology: LL and ZL; investigation: FW, ZL, XN, LZ, JZ, and LQ; formal analysis: YS; and writing (review and editing): FW, ZL, XN, LZ, JZ, LQ, LL, ZL, YS, LD, JL, YZ, XW, and HL.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, F., Liu, Z., Niu, X. et al. Safety and Pharmacokinetics of PSD502 in Healthy Chinese Male and Female Volunteers: Two Randomized, Double-Blind, Placebo-Controlled, Phase I Trials. Clin Drug Investig 43, 503–515 (2023). https://doi.org/10.1007/s40261-023-01277-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01277-4