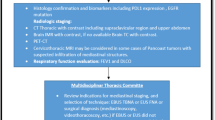
Abstract
Background and Objective
Checkpoint inhibitor-related pneumonitis (CIP) is one of the most common serious and fatal adverse events associated with immune checkpoint inhibitors (ICIs). The study sought to identify risk factors of all-grade and severe CIP and to construct a risk-scoring model specifically for severe CIP.
Methods
This observational, retrospective case–control study involved 666 lung cancer patients who received ICIs between April 2018 and March 2021. The study analyzed patient demographic, preexisting lung diseases, and the characteristics and treatment of lung cancer to determine the risk factors for all-grade and severe CIP. A risk score for severe CIP was developed and validated in a separate patient cohort of 187 patients.
Results
Among 666 patients, 95 patients were afflicted with CIP, of which 37 were severe cases. Multivariate analysis revealed age ≥ 65 years, current smoking, chronic obstructive pulmonary disease, squamous cell carcinoma, prior thoracic radiotherapy, and extra-thoracic radiotherapy during ICI were independently associated with CIP events. Five factors, emphysema (odds ratio [OR] 2.87), interstitial lung disease (OR 4.76), pleural effusion (OR 3.00), history of radiotherapy during ICI (OR 4.30), and single-agent immunotherapy (OR 2.44) were independently associated with severe CIP and were incorporated into a risk-score model (score ranging 0–17). The area under the model receiver operating characteristic curve for the model was 0.769 in the development cohort and 0.749 in the validation cohort.
Conclusions
The simple risk-scoring model may predict severe CIP in lung cancer patients receiving ICIs. For patients with high scores, clinicians should use ICIs with caution or strengthen the monitoring of these patients.


Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Khan M, Lin J, Liao G, et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97: e11936.
Tan PS, Aguiar P Jr, Haaland B, et al. Comparative effectiveness of immune-checkpoint inhibitors for previously treated advanced non-small cell lung cancer—a systematic review and network meta-analysis of 3024 participants. Lung Cancer. 2018;115:84–8.
Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91.
Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8.
Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154:1416–23.
Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–81.
Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–6.
Atchley WT, Alvarez C, Saxena-Beem S, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in North Carolina. Chest. 2021;160:731–42.
Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22:6051–60.
Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13:1930–9.
Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–17.
Lin X, Deng H, Chen L, et al. Clinical types of checkpoint inhibitor-related pneumonitis in lung cancer patients: a multicenter experience. Transl Lung Cancer Res. 2021;10:415–29.
Cui P, Huang D, Wu Z, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol. 2020;12:1758835920922033.
Tone M, Izumo T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer. 2019;10:2006–12.
Zhou C, Yang Y, Lin X, et al. Proposed clinical phases for the improvement of personalized treatment of checkpoint inhibitor-related pneumonitis. Front Immunol. 2022;13: 935779.
Mennini FS, Bini C, Marcellusi A, Del Vecchio M. Cost estimate of immune-related adverse reactions associated with innovative treatments of metastatic melanoma. Clin Drug Investig. 2018;38:967–76.
Shibaki R, Murakami S, Matsumoto Y, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother. 2020;69:15–22.
Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903.
Lin X, Deng H, Yang Y, et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front Oncol. 2021;11: 698832.
Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1607–16.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51.
Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–5.
Wang YN, Lou DF, Li DY, et al. Elevated levels of IL-17A and IL-35 in plasma and bronchoalveolar lavage fluid are associated with checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer. Oncol Lett. 2020;20:611–22.
Agalioti T, Giannou AD, Stathopoulos GT. Pleural involvement in lung cancer. J Thorac Dis. 2015;7:1021–30.
Ye ZJ, Zhou Q, Gu YY, et al. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion [published correction appears in J Immunol. 2014 Nov 1;193(9):4748]. J Immunol. 2010;185:6348–54.
Lin H, Tong ZH, Xu QQ, et al. Interplay of Th1 and Th17 cells in murine models of malignant pleural effusion. Am J Respir Crit Care Med. 2014;189:697–706.
Asada M, Mikami T, Niimura T, et al. The risk factors associated with immune checkpoint inhibitor-related pneumonitis. Oncology. 2021;99:256–9.
Suazo-Zepeda E, Bokern M, Vinke PC, et al. Risk factors for adverse events induced by immune checkpoint inhibitors in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Immunol Immunother. 2021;70:3069–80.
Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91:142–9.
Cheng LL, Liu YY, Su ZQ, et al. Clinical characteristics of tobacco smoke-induced versus biomass fuel-induced chronic obstructive pulmonary disease. J Transl Int Med. 2015;3:126–9.
Li L, Yang DC, Chen CH. Metabolic reprogramming: a driver of cigarette smoke-induced inflammatory lung diseases. Free Radic Biol Med. 2021;163:392–401.
Galant-Swafford J, Troesch A, Tran L, Weaver A, Doherty TA, Patel SP. Landscape of immune-related pneumonitis in cancer patients with asthma being treated with immune checkpoint blockade. Oncology. 2020;98:123–30.
Barrón F, Sánchez R, Arroyo-Hernández M, et al. Risk of developing checkpoint immune pneumonitis and its effect on overall survival in non-small cell lung cancer patients previously treated with radiotherapy. Front Oncol. 2020;10: 570233.
Kalisz KR, Ramaiya NH, Laukamp KR, et al. Immune checkpoint inhibitor therapy-related pneumonitis: patterns and management. Radiographics. 2019;39:1923–37.
Xu Z, Feng J, Weng Y, et al. Combination of immune checkpoint inhibitors and radiotherapy for advanced non-small-cell lung cancer and prostate cancer: a meta-analysis. J Oncol. 2021;2021:6631643.
Tian S, Switchenko JM, Buchwald ZS, et al. Lung stereotactic body radiation therapy and concurrent immunotherapy: a multicenter safety and toxicity analysis. Int J Radiat Oncol Biol Phys. 2020;108:304–13.
Sul J, Blumenthal GM, Jiang X, et al. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist. 2016;21:643–50.
Chao Y, Zhou J, Hsu S, et al. Risk factors for immune checkpoint inhibitor-related pneumonitis in non-small cell lung cancer. Transl Lung Cancer Res. 2022;11:295–306.
Caramori G, Ruggeri P, Mumby S, et al. Molecular links between COPD and lung cancer: new targets for drug discovery? Expert Opin Ther Targets. 2019;23:539–53.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68.
Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing interstitial lung disease is associated with onset of nivolumab-induced pneumonitis in patients with solid tumors: a retrospective analysis. BMC Cancer. 2021;21:924.
Yamaguchi T, Shimizu J, Oya Y, et al. Risk factors for pneumonitis in patients with non-small cell lung cancer treated with immune checkpoint inhibitors plus chemotherapy: a retrospective analysis. Thorac Cancer. 2022;13:724–31.
Reuss JE, Brigham E, Psoter KJ, et al. Pretreatment lung function and checkpoint inhibitor pneumonitis in NSCLC. JTO Clin Res Rep. 2021;2: 100220.
Qu Y, Cheng B, Shao N, et al. Prognostic value of immune-related genes in the tumor microenvironment of lung adenocarcinoma and lung squamous cell carcinoma. Aging (Albany NY). 2020;12:4757–77.
Lin X, Deng J, Deng H, et al. Comprehensive analysis of the immune microenvironment in checkpoint inhibitor pneumonitis. Front Immunol. 2022;12: 818492.
Chen X, Zhang Z, Hou X, et al. Immune-related pneumonitis associated with immune checkpoint inhibitors in lung cancer: a network meta-analysis. J Immunother Cancer. 2020;8: e001170.
Iwai T, Sugimoto M, Patel H, et al. Anti-VEGF antibody protects against alveolar exudate leakage caused by vascular hyperpermeability, resulting in mitigation of pneumonitis induced by immunotherapy. Mol Cancer Ther. 2021;20:2519–26.
Yamakawa H, Oba T, Ohta H, et al. Nintedanib allows retreatment with atezolizumab of combined non-small cell lung cancer/idiopathic pulmonary fibrosis after atezolizumab-induced pneumonitis: a case report. BMC Pulm Med. 2019;19:156.
Zhang B, Wu Q, Zhou YL, et al. Immune-related adverse events from combination immunotherapy in cancer patients: a comprehensive meta-analysis of randomized controlled trials. Int Immunopharmacol. 2018;63:292–8.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by the National Key R&D Program of China (Grant number: 2021YFC2301101), State Key Laboratory of Respiratory Disease-The open project (Grant number: SKLRD-OP-202011, SKLRD-OP-202111), the Fundamental and Applied Fundamental Research Project of City-School (Institute) Joint Funding Project, Guangzhou Science and Technology Bureau (Grant number: 202102010186), and Beijing Xisike Clinical Oncology Research Foundation (Grant number: Y-HS202102-0118).
Conflict of interest
The authors report no conflict of interest.
Ethics approval
Approval was granted by the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Code availability
Not applicable.
Author contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by HD, JD, XL, WG, ZL, YQ, YY, JW, GQ, NS, and MZ. The first draft of the manuscript was written by HD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, H., Deng, J., Lin, X. et al. A Risk-Scoring Model for Severe Checkpoint Inhibitor-Related Pneumonitis: A Case–Control Study. Clin Drug Investig 43, 347–357 (2023). https://doi.org/10.1007/s40261-023-01267-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01267-6