Abstract
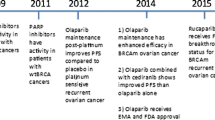
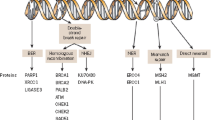
As a drug class, inhibitors of poly-(ADP-ribose) polymerase (PARP) have had their greatest impact on the treatment of women with epithelial ovarian cancers (EOC), in particular, those with the most common histological subtype, high-grade serous cancer, as it has high rates of homologous recombination (HR) deficiency. PARP inhibition exploits this cancer vulnerability by further disrupting DNA repair, thus leading to genomic catastrophe. Early clinical data demonstrated the effectiveness of PARP inhibition in women with recurrent EOC harbouring BRCA1/2 mutations and those with platinum-sensitive recurrences. Three PARP inhibitors (olaparib, niraparib, and rucaparib) are now approved for use in women with recurrent EOC. Based upon randomised controlled trials, PARP inhibitors are in use as “maintenance” therapy for those with platinum-sensitive and platinum-responsive recurrences (irrespective of BRCA1/2 mutation status). Among women with BRCA1/2 mutations (either germline or somatic), maintenance PARP inhibitor therapy for those with recurrence has led to a nearly fourfold prolongation of progression-free survival compared to placebo control. Those without BRCA1/2 mutations experience an approximately twofold increase in progression-free survival. The latest clinical data demonstrate that women with BRCA1/2 mutations who respond to first-line chemotherapy and go on to have maintenance olaparib experience a doubling of the rate of freedom from death at 3 years when compared to placebo (60% vs 27%). PARP inhibitors are also approved as active therapy for women with germline or tumour BRCA1/2 mutations and recurrent EOC treated with three or more prior lines of therapy. Apart from the presence of a BRCA1/2 mutation (germline or somatic) and clinical factors such as platinum sensitivity and responsiveness, other predictive biomarkers are not in routine clinical use. Assays to identify genomic aberrations caused by HR deficiency, or mutations in genes involved in HR, have not been sufficiently sensitive to identify all patients who benefit from treatment. The mechanisms of PARP-inhibitor resistance include restoration of HR through reversion mutations in HR genes, capable of re-establishing the DNA open-reading frame and leading to resumed HR function. Other mechanisms that sustain sufficient DNA repair may also be important. This review focuses on the rationale for the use of PARP inhibitors in EOC. The data that have shaped clinical research are presented, and the trials that have changed management standards are reviewed and discussed. Highlighted are the past and ongoing efforts to further improve and explore the use of PARP inhibitors in EOC.

Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. https://doi.org/10.3322/caac.21387.
Chen Y, Du H. The promising PARP inhibitors in ovarian cancer therapy: from olaparib to others. Biomed Pharmacother. 2018;99:552–60. https://doi.org/10.1016/j.biopha.2018.01.094.
Kurman RJ, Carcangiu ML, Herrington CS. World Health Organisation classification of tumours of the female reproductive organs. 4th revised ed. International Agency for Research on Cancer; 2014.
Singh N, McCluggage WG, Gilks CB. High-grade serous carcinoma of tubo-ovarian origin: recent developments. Histopathology. 2017;71(3):339–56. https://doi.org/10.1111/his.13248.
Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. https://doi.org/10.1002/cncr.21536.
Hennessy BTJ, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28(22):3570–6. https://doi.org/10.1200/JCO.2009.27.2997.
Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–63. https://doi.org/10.1200/JCO.2011.39.8545.
Ledermann JA. Lessons learned from a decade of clinical trials of high-dose chemotherapy in ovarian cancer. Int J Gynecol Cancer. 2008;18(Suppl 1):53–8. https://doi.org/10.1111/j.1525-1438.2007.01107.x.
Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14(10):1020–6. https://doi.org/10.1016/S1470-2045(13)70363-2.
Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. https://doi.org/10.1056/NEJMoa052985.
Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96. https://doi.org/10.1056/NEJMoa1103799.
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. https://doi.org/10.1056/NEJMoa1104390.
Atlas CGAR, Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. https://doi.org/10.1038/nature10166.
Ashworth A, Lord CJ. Synthetic lethal therapies for cancer: what’s next after PARP inhibitors? Nat Rev Clin Oncol. 2018;15(9):564–76. https://doi.org/10.1038/s41571-018-0055-6.
Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26(22):3785–90. https://doi.org/10.1200/JCO.2008.16.0812.
Ohmoto A, Yachida S. Current status of poly(ADP-ribose) polymerase inhibitors and future directions. Onco Targets Ther. 2017;10:5195–208. https://doi.org/10.2147/OTT.S139336.
Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24(17):4062–5. https://doi.org/10.1158/1078-0432.CCR-18-1314.
Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49–58. https://doi.org/10.1016/j.ejca.2016.03.005.
D’Andrea AD. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair (Amst). 2018. https://doi.org/10.1016/j.dnarep.2018.08.021.
Meghani K, Fuchs W, Detappe A, et al. Multifaceted impact of microRNA 493-5p on genome-stabilizing pathways induces platinum and PARP inhibitor resistance in BRCA2-mutated carcinomas. Cell Rep. 2018;23(1):100–11. https://doi.org/10.1016/j.celrep.2018.03.038.
Khan OA, Gore M, Lorigan P, et al. A phase I study of the safety and tolerability of olaparib (AZD2281, KU0059436) and dacarbazine in patients with advanced solid tumours. Br J Cancer. 2011;104(5):750–5. https://doi.org/10.1038/bjc.2011.8.
Samol J, Ranson M, Scott E, et al. Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Invest New Drugs. 2012;30(4):1493–500. https://doi.org/10.1007/s10637-011-9682-9.
Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. https://doi.org/10.1056/NEJMoa0900212.
Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–9. https://doi.org/10.1200/JCO.2009.26.9589.
Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61. https://doi.org/10.1016/S1470-2045(11)70214-5.
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. https://doi.org/10.1056/NEJMoa1105535.
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–61. https://doi.org/10.1016/S1470-2045(14)70228-1.
Ledermann JA, Harter P, Gourley C, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17(11):1579–89. https://doi.org/10.1016/S1470-2045(16)30376-X.
Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84. https://doi.org/10.1016/S1470-2045(17)30469-2.
Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64. https://doi.org/10.1056/NEJMoa1611310.
Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–61. https://doi.org/10.1016/S0140-6736(17)32440-6.
Ledermann JA, Harter P, Gourley C, et al. Quality of life during olaparib maintenance therapy in platinum-sensitive relapsed serous ovarian cancer. Br J Cancer. 2016;115:1313–20. https://doi.org/10.1038/bjc.2016.348.
Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018;19:1126–34. https://doi.org/10.1016/S1470-2045(18)30343-7.
Oza AM, Matulonis UA, Malander S, et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2018;19:1117–25. https://doi.org/10.1016/S1470-2045(18)30333-4.
Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505. https://doi.org/10.1056/nejmoa1810858.
Dougherty BA, Lai Z, Hodgson DR, et al. Biological and clinical evidence for somatic mutations in BRCA1 and BRCA2 as predictive markers for olaparib response in high-grade serous ovarian cancers in the maintenance setting. Oncotarget. 2017;8(27):43653–61. https://doi.org/10.18632/oncotarget.17613.
Oza AM, Tinker AV, Oaknin A, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol. 2017;147(2):267–75. https://doi.org/10.1016/j.ygyno.2017.08.022.
den Brok WD, Schrader KA, Sun S, et al. Homologous recombination deficiency in breast cancer: a clinical review. JCO Precis Oncol. 2017;1:1–13. https://doi.org/10.1200/PO.16.00031.
Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50. https://doi.org/10.1200/JCO.2014.56.2728.
Coleman RL, Sill MW, Bell-McGuinn K, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation—an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137(3):386–91. https://doi.org/10.1016/j.ygyno.2015.03.042.
Dal Molin GZ, Omatsu K, Sood AK, Coleman RL. Rucaparib in ovarian cancer: an update on safety, efficacy and place in therapy. Ther Adv Med Oncol. 2018;10:1758835918778483. https://doi.org/10.1177/1758835918778483.
Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–9. https://doi.org/10.1200/JCO.2011.36.9215.
Kristeleit R, Shapiro GI, Burris HA, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2 -mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23(15):4095–106. https://doi.org/10.1158/1078-0432.CCR-16-2796.
Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87. https://doi.org/10.1016/S1470-2045(16)30559-9.
Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16(1):87–97. https://doi.org/10.1016/S1470-2045(14)71135-0.
US National Library of Medicine. Veliparib with carboplatin and paclitaxel and as continuation maintenance therapy in subjects with newly diagnosed stage III or IV, high-grade serous, epithelial ovarian, fallopian tube, or primary peritoneal cancer; 2019. https://clinicaltrials.gov/ct2/show/NCT02470585. Accessed 12 Mar 2019.
Oza AM, Lorusso D, Oaknin A, et al. ARIEL4: an international, multicenter randomized phase 3 study of the PARP inhibitor rucaparib vs chemotherapy in germline or somatic BRCA1- or BRCA2- mutated, relapsed, high-grade ovarian carcinoma. J Clin Oncol. 2017. https://doi.org/10.1200/jco.2017.35.15_suppl.tps5603.
Lowe ES, Jayawardene D, Penson RT. SOLO3: a randomized phase III trial of olaparib versus chemotherapy in platinum-sensitive relapsed ovarian cancer patients with a germline BRCA1/2 mutation (gBRCAm). J Clin Oncol. 2016. https://doi.org/10.1200/jco.2016.34.15_suppl.tps5598.
Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149(1):214–20. https://doi.org/10.1016/j.ygyno.2018.01.011.
Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–51. https://doi.org/10.1016/S0140-6736(10)60893-8.
Incorvaia L, Passiglia F, Rizzo S, et al. “Back to a false normality”: new intriguing mechanisms of resistance to PARP inhibitors. Oncotarget. 2017;8(14):23891–904. https://doi.org/10.18632/oncotarget.14409.
Kondrashova O, Nguyen M, Shield-Artin K, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP Inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7(9):984–98. https://doi.org/10.1158/2159-8290.CD-17-0419.
Domchek SM. Reversion mutations with clinical use of PARP inhibitors: many genes, many versions. Cancer Discov. 2017;7(9):937–9. https://doi.org/10.1158/2159-8290.CD-17-0734.
Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–5. https://doi.org/10.1038/nature06548.
Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451(7182):1116–20. https://doi.org/10.1038/nature06633.
Barber LJ, Sandhu S, Chen L, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229(3):422–9. https://doi.org/10.1002/path.4140.
Norquist B, Wurz KA, Pennil CC, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29(22):3008–15. https://doi.org/10.1200/JCO.2010.34.2980.
Christie EL, Fereday S, Doig K, Pattnaik S, Dawson S-J, Bowtell DDL. Reversion of BRCA1/2 germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J Clin Oncol. 2017;35(12):1274–80. https://doi.org/10.1200/JCO.2016.70.4627.
Johnson N, Johnson SF, Yao W, et al. Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance. Proc Natl Acad Sci USA. 2013;110(42):17041–6. https://doi.org/10.1073/pnas.1305170110.
Drost R, Bouwman P, Rottenberg S, et al. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer Cell. 2011;20(6):797–809. https://doi.org/10.1016/j.ccr.2011.11.014.
Bunting SF, Callén E, Wong N, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141(2):243–54. https://doi.org/10.1016/j.cell.2010.03.012.
Bouwman P, Aly A, Escandell JM, et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol. 2010;17(6):688–95. https://doi.org/10.1038/nsmb.1831.
Ray Chaudhuri A, Callen E, Ding X, et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature. 2016;535(7612):382–7. https://doi.org/10.1038/nature18325.
Rondinelli B, Gogola E, Yücel H, et al. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat Cell Biol. 2017;19(11):1371–8. https://doi.org/10.1038/ncb3626.
Clements KE, Thakar T, Nicolae CM, Liang X, Wang H-G, Moldovan G-L. Loss of E2F7 confers resistance to poly-ADP-ribose polymerase (PARP) inhibitors in BRCA2-deficient cells. Nucleic Acids Res. 2018;46(17):8898–907. https://doi.org/10.1093/nar/gky657.
Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105(44):17079–84. https://doi.org/10.1073/pnas.0806092105.
Du Y, Yamaguchi H, Wei Y, et al. Blocking c-Met–mediated PARP1 phosphorylation enhances anti-tumor effects of PARP inhibitors. Nat Med. 2016;22(2):194–201. https://doi.org/10.1038/nm.4032.
Choi YE, Meghani K, Brault M-E, et al. Platinum and PARP inhibitor resistance due to overexpression of microRNA-622 in BRCA1-mutant ovarian cancer. Cell Rep. 2016;14(3):429–39. https://doi.org/10.1016/j.celrep.2015.12.046.
Nih U.S. National Library of Medicine. A study of long-term responders on olaparib (OLALA). 2019. https://clinicaltrials.gov/ct2/show/NCT02489058. Published 2018. Accessed 14 Feb 2019.
US National Library of Medicine. Platine, Avastin and OLAparib in 1st line (PAOLA 1). 2019. https://clinicaltrials.gov/ct2/show/NCT02477644. Accessed 12 Mar 2019.
US National Library of Medicine. A study of maintenance niraparib treatment in patients with advanced ovarian cancer following response on front-line platinum-based chemotherapy. 2019. https://clinicaltrials.gov/ct2/show/NCT02655016. Accessed 12 Mar 2019.
US National Library of Medicine. A phase 3 comparison of platinum-based therapy with TSR-042 and niraparib versus standard of care platinum-based therapy as first-line treatment of stage III or IV nonmucinous epithelial ovarian cancer (FIRST). 2019. https://clinicaltrials.gov/ct2/show/NCT03806049. Accessed 12 Mar 2019.
US National Library of Medicine. Study evaluating the efficacy of maintenance olaparib and cediranib or olaparib alone in ovarian cancer patients (ICON9). 2018. https://clinicaltrials.gov/ct2/show/NCT03278717. Published 2018. Accessed 13 Oct 2018.
US National Library of Medicine. Platinum-based chemotherapy with atezolizumab and niraparib in patients with recurrent ovarian cancer (ANITA). 2018. https://clinicaltrials.gov/ct2/show/NCT03598270?term=NIRAPARIB&cond=Ovarian+Cancer&draw=2&rank=12. Published 2018. Accessed 13 Oct 2018.
US National Library of Medicine. A study in ovarian cancer patients evaluation rucaparib and nivolumab as maintenance treatment following response to front-line platinum-based chemotherapy (ATHENA). 2018. https://clinicaltrials.gov/ct2/show/NCT03522246?term=RUCAPARIB&cond=Ovarian+Cancer&rank=3. Published 2018. Accessed 13 Oct 2018.
US National Library of Medicine. Avelumab and talazoparib in untreated advanced ovarian cancer (JAVELIN OVARIAN PARP 100). 2019. https://clinicaltrials.gov/ct2/show/NCT03642132. Accessed 12 Mar 2019.
US National Library of Medicine. Cediranib maleate and olaparib or standard chemotherapy in treating patients with recurrent platinum-resistant or -refractory ovarian, fallopian tube, or primary peritoneal cancer. Clinical Trials. 2018. https://clinicaltrials.gov/ct2/show/NCT02502266. Published 2015. Accessed 13 Oct 2018.
Kummar S, Oza AM, Fleming GF, et al. Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res. 2015;21(7):1574–82. https://doi.org/10.1158/1078-0432.CCR-14-2565.
US National Library of Medicine. Olaparib or cediranib maleate and olaparib compared with standard platinum-based chemotherapy in treating patients with recurrent platinum-sensitive ovarian, fallopian tube, or primary peritoneal cancer. 2019. https://clinicaltrials.gov/ct2/show/NCT02446600. Accessed 12 Mar 2019.
US National Library of Medicine. A study of rucaparib versus chemotherapy BRCA mutant ovarian, fallopian tube, or primary peritoneal cancer patients. 2019. https://clinicaltrials.gov/ct2/show/NCT02855944. Accessed 12 Mar 2019.
US National Library of Medicine. Olaparib treatment in relapsed germline breast cancer susceptibility gene (BRCA) mutated ovarian cancer patients who have progressed at least 6 months after last platinum treatment and have received at least 2 prior platinum treatments (SOLO 3). 2018. https://clinicaltrials.gov/ct2/show/NCT02282020. Accessed 13 Oct 2018.
US National Library of Medicine. Recurrent ovarian carcinosarcoma anti-pd-1 niraparib (ROCSAN). 2019. https://clinicaltrials.gov/ct2/show/NCT02855944. Accessed 12 Mar 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this review.
Conflicts of Interest
Anna Tinker has received research grant funding and honoraria from AstraZeneca. Sarah Cook has no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Cook, S.A., Tinker, A.V. PARP Inhibitors and the Evolving Landscape of Ovarian Cancer Management: A Review. BioDrugs 33, 255–273 (2019). https://doi.org/10.1007/s40259-019-00347-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-019-00347-4