Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a highly prevalent chronic respiratory disease with considerable clinical and socioeconomic impact. Budesonide/glycopyrrolate/formoterol fumarate (BGF) is a newly approved pharmacotherapy for COPD in China that has been shown to improve lung function and reduce the risk of exacerbations, but the cost-effectiveness of BGF remains unknown. The objective of this study was to evaluate the cost-effectiveness of BGF in patients with moderate to very severe COPD from a Chinese healthcare system perspective.
Methods
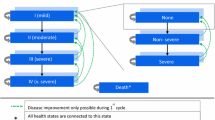
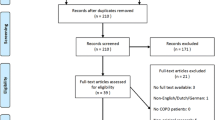
A semi-Markov model was developed to compare the costs and benefit of treatment with BGF versus a composite comparator of long-acting muscarinic antagonist/long-acting β2-agonist (LAMA/LABA) therapies. Clinical inputs for BGF and the composite comparator were based on the KRONOS study (NCT02497001) and a network meta-analysis. Cost inputs were derived from published literature and Chinese government documents, supplemented by expert opinion where necessary. Health-related quality-of-life inputs were also obtained based on the KRONOS study. Lifetime costs, number of exacerbations, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs) were estimated. Costs were measured in 2020 Chinese Yuan (CN¥) and converted into US dollars (US$). Scenario analyses and sensitivity analyses were conducted.
Results
Over the lifetime horizon, BGF treatment led to fewer moderate and severe exacerbations (4.01 and 0.87, respectively) versus the composite comparator (8.42 and 2.04, respectively), with a base-case ICER of CN¥13,685.94 (US$1983.47) per QALY gained. Scenario analyses considering different population and utilities resulted in ICERs ranging from dominant to CN¥13,673.91 (US$1981.73). Extensive sensitivity analyses indicated robust base-case results since all analyses yielded ICERs below the conservative cost-effectiveness threshold of one times the Chinese per capita gross domestic product (CN¥72,447.00 [US$10,499.57], 2020).
Conclusion
Triple therapy with BGF was predicted to improve outcomes and be a cost-effective treatment option compared with LAMA/LABA therapies for patients with moderate to very severe COPD in China.




Similar content being viewed by others
References
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD; 2020. http://goldcopd.org. Accessed 31 Mar 2021.
Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20(1):11–23.
Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–17.
Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–58.
Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–30.
Chronic Obstructive Pulmonary Disease Group of the Society of Respiratory Diseases of the Chinese Medical Association, Chronic Obstructive Pulmonary Disease Working Committee of Respiratory Diseases of Chinese Medical Doctor Association. Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021). Chin J Tuberc Respir Dis. 2021;44(03):170–205.
Ding B, Small M, Holmgren U. A cross-sectional survey of current treatment and symptom burden of patients with COPD consulting for routine care according to GOLD 2014 classifications. Int J Chron Obstruct Pulmon Dis. 2017;12:1527–37.
Doty A, Schroeder J, Vang K, et al. Drug delivery from an innovative LAMA/LABA co-suspension delivery technology fixed-dose combination MDI: evidence of consistency, robustness, and reliability. AAPS PharmSciTech. 2018;19(2):837–44.
Taylor G, Warren S, Dwivedi S, et al. Gamma scintigraphic pulmonary deposition study of glycopyrronium/formoterol metered dose inhaler formulated using co-suspension delivery technology. Eur J Pharm Sci. 2018;111:450–7.
Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–58.
Lakhotia B, Mahon R, Gutzwiller FS, Danyliv A, Nikolaev I, Thokala P. Modelling the cost-effectiveness of indacaterol/glycopyrronium versus salmeterol/fluticasone using a novel Markov exacerbation-based approach. Int J Chron Obstruct Pulmon Dis. 2020;15:787–97.
Ramos M, Lamotte M, Gerlier L, Svangren P, Miquel-Cases A, Haughney J. Cost-effectiveness of physical activity in the management of COPD patients in the UK. Int J Chron Obstruct Pulmon Dis. 2019;14:227–39.
Rajagopalan K, Bloudek L, Marvel J, Dembek C, Kavati A. Cost-effectiveness of twice-daily indacaterol/glycopyrrolate inhalation powder for the treatment of moderate to severe COPD in the US. Int J Chron Obstruct Pulmon Dis. 2018;13:3867–77.
Capel M, Mareque M, Alvarez CJ, Lindner L, Oyaguez I. Cost-effectiveness of fixed-dose combinations therapies for chronic obstructive pulmonary disease treatment. Clin Drug Investig. 2018;38(7):611–20.
Wilson MR, Patel JG, Coleman A, McDade CL, Stanford RH, Earnshaw SR. Cost-effectiveness analysis of umeclidinium/vilanterol for the management of patients with moderate to very severe COPD using an economic model. Int J Chron Obstruct Pulmon Dis. 2017;12:997–1008.
Selya-Hammer C, Gonzalez-Rojas Guix N, Baldwin M, et al. Development of an enhanced health-economic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat® fixed-dose combination for chronic obstructive pulmonary disease patients in Italy. Ther Adv Respir Dis. 2016;10(5):391–401.
Ramos M, Haughney J, Henry N, Lindner L, Lamotte M. Cost versus utility of aclidinium bromide 400 microg plus formoterol fumarate dihydrate 12 microg compared to aclidinium bromide 400 microg alone in the management of moderate-to-severe COPD. Clinicoecon Outcomes Res. 2016;8:445–56.
Costa-Scharplatz M, Stallberg B, Goyal P, Asukai Y, Gruenberger JB, Price D. Cost-effectiveness of glycopyrronium bromide compared with tiotropium in patients with chronic obstructive pulmonary disease in Sweden. Appl Health Econ Health Policy. 2015;13(6):637–45.
Asukai Y, Baldwin M, Fonseca T, Gray A, Mungapen L, Price D. Improving clinical reality in chronic obstructive pulmonary disease economic modelling: development and validation of a micro-simulation approach. Pharmacoeconomics. 2013;31(2):151–61.
Rutten-van Molken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ. 2007;8(2):123–35.
Liu GHS, Wu J, et al. China guidelines for pharmacoeconomic evaluations. Beijing: China Market Press; 2020.
AstraZeneca. China LAMA/LABA market share data (data on file). AstraZeneca; 2020.
Siddiqui MK, Shukla P, Jenkins M, et al. Systematic review and network meta-analysis of the efficacy and safety of glycopyrrolate/formoterol fumarate metered dose inhaler in comparison with other long-acting muscarinic antagonist/long-acting beta2-agonist fixed-dose combinations in COPD. Ther Adv Respir Dis. 2019;13:1753466619894502.
Zhang J, Zheng J, Huang K, Chen Y, Yang J, Yao W. Use of glucocorticoids in patients with COPD exacerbations in China: a retrospective observational study. Ther Adv Respir Dis. 2018;12:1753466618769514.
Department of Population and Employment Statistics of the National Bureau of Statistics of China. China population and employment statistics yearbook 2019. Beijing: China Statistics Press; 2019.
Lan F, Li J, Yu C, et al. Associations between airflow obstruction and total and cause-specific mortality in adults in China. Chin J Epidemiol. 2017;38(1):13–9.
National Healthcare Security Administration. Notice on integrating the drugs negotiated in 2019 into category B of the national reimbursement drug list of basic medical insurance, industrial injury insurance and maternity insurance by National Healthcare Security Administration and Ministry of Human Resources and Social Security; 2019. http://www.nhsa.gov.cn/art/2019/11/28/art_37_2050.html. Accessed 31 Mar 2021.
YaoZh. Inquiry of drug bid information. https://www.yaozh.com. Accessed 31 Mar 2021.
National Healthcare Security Administration. Policy interpretation of Notice on printing and distributing the national reimbursement drug list of basic medical insurance, industrial injury insurance and maternity insurance (2020) by National Healthcare Security Administration and Ministry of Human Resources and Social Security; 2020. http://www.nhsa.gov.cn/art/2020/12/28/art_38_4219.html. Accessed 31 Mar 2021.
National Healthcare Security Administration. Policy interpretation of Notice on integrating the drugs negotiated in 2019 into category B of the national reimbursement drug list of basic medical insurance, industrial injury insurance and maternity insurance by National Healthcare Security Administration and Ministry of Human Resources and Social Security; 2019. http://www.nhsa.gov.cn/art/2019/11/28/art_38_2056.html. Accessed 31 Mar 2021.
Liaoning Provincial Development and Reform Commission; Health Commission of Liaoning Province; Liaoning Provincial Department of Human Resources and Social Security; Liaoning Province Finance Department. Notice on printing and distributing price of medical service projects of public medical institutions in Liaoning Province. http://wsjk.ln.gov.cn/wst_wjgg/201710/t20171027_3092708.html. Accessed 31 Mar 2021.
Chengdu Healthcare Security Administration; Chengdu Municipal Health Commission. Price list of medical service price structure adjustment projects of municipal public medical institutions in Chengdu. http://cdyb.chengdu.gov.cn/ylbzj/c128998/2020-12/02/content_ab17741660454da38fa5181ae88b2877.shtml. Accessed 31 Mar 2021.
People’s Government of Guangdong Province. Medical service projects and prices of public medical institutions in Guangzhou. http://gddata.gd.gov.cn/data/dataSet/toDataDetails/29000_02700765. Accessed 31 Mar 2021.
Shanghai Municipal Development and Reform Commission. Medical service price inquiry. http://fgw.sh.gov.cn/jggl/. Accessed 31 Mar 2021.
Beijing Municipal Medical Insurance Bureau. Medical service price inquiry. http://ybj.beijing.gov.cn/2020_zwfw/2020_bmcx/. Accessed 31 Mar 2021.
National Bureau of Statistics of China. Statistical bulletin of national economic and social development of the people's Republic of China in 2020. http://www.stats.gov.cn/tjsj/zxfb/202102/t20210227_1814154.html. Accessed 31 Mar 2021.
Samyshkin Y, Schlunegger M, Haefliger S, Ledderhose S, Radford M. Cost-effectiveness of roflumilast in combination with bronchodilator therapies in patients with severe and very severe COPD in Switzerland. Int J Chron Obstruct Pulmon Dis. 2013;8:79–87.
Luo N, Liu G, Li M, et al. Estimating an EQ-5D-5L value set for China. Value Health. 2017;20(4):662–9.
Liu GG, Wu H, Li M, et al. Chinese time trade-off values for EQ-5D health states. Value Health. 2014;17(5):597–604.
Starkie HJ, Briggs AH, Chambers MG, Jones P. Predicting EQ-5D values using the SGRQ. Value Health. 2011;14(2):354–60.
Ismaila AS, Risebrough N, Schroeder M, et al. Cost-effectiveness of once-daily single-inhaler triple therapy in COPD: the IMPACT trial. Int J Chron Obstruct Pulmon Dis. 2019;14:2681–95.
Acknowledgements
The authors thank Enrico de Nigris from AstraZeneca, Cambridge, UK, for his support and advice on the modeling methodology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by AstraZeneca Pharmaceutical Co. Ltd (Beijing, China).
Conflict of interest
Jia Liu, Xiaoning He, and Jing Wu declare they have no conflicts of interest.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript. The Microsoft Excel simulation model used for the analysis is available from the corresponding author upon reasonable request.
Author contributions
Concept and design: JL, XH, and JW. Acquisition of data: JL, XH, and JW. Development of decision analytical model: JL, XH, and JW. Analysis of data: JL. All authors participated in critically reviewing and interpreting the data, critically reviewed the manuscript for intellectual content, and approved the final version for publication submission. All authors agree to be accountable for all aspects of this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., He, X. & Wu, J. Economic Evaluation of Triple Therapy with Budesonide/Glycopyrrolate/Formoterol Fumarate for the Treatment of Moderate to Very Severe Chronic Obstructive Pulmonary Disease in China Using a Semi-Markov Model. Appl Health Econ Health Policy 20, 743–755 (2022). https://doi.org/10.1007/s40258-022-00732-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00732-1