Abstract
Background
Chronic lymphocytic leukaemia (CLL) mostly affects patients with comorbidities and limited therapeutic options. Obinutuzumab in combination with chlorambucil (GClb) is a new therapeutic option for previously untreated CLL patients who are unsuitable for full-dose fludarabine-based therapy. This combination delays disease progression but incurs additional costs; thus, an assessment of its value for money is relevant.
Objective
To estimate the incremental cost-utility ratio of GClb in comparison with (i) rituximab in combination with chlorambucil (RClb), and (ii) chlorambucil alone (Clb) from the perspective of the Portuguese National Health Service (NHS).
Methods
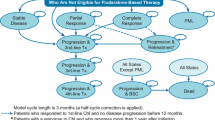
A Markov model was used to predict disease progression. Pre‐progression clinical data were based on the latest CLL11 trial data, and post‐progression clinical data were obtained from CLL5 trial data. Utility values are from Kosmas et al. (Leuk Lymphoma 56:1320–1326, 14). Only direct medical costs were included. The resource consumption was estimated by a panel of Portuguese experts, and the unit costs were obtained from official sources. A discount rate of 5% was applied to costs and consequences.
Results
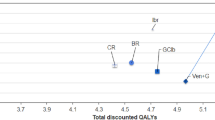
GClb and RClb were associated with an increase of 1.06 and 0.39 quality-adjusted life-years (QALY) at an additional cost of €21,720 and €9836 when compared to Clb, respectively. The cost-utility ratio of GClb versus Clb was €20,397/QALY, while RClb was extendedly dominated.
Conclusions
The use of GClb for previously untreated CLL patients who are unsuitable for full-dose fludarabine-based therapy incurs an incremental cost per QALY that is generally accepted in Portugal. Therefore, although there is some uncertainty, obinutuzumab is probably a cost-effective therapy in the Portuguese setting.





Similar content being viewed by others
References
Watson L, Wyld P, Catovsky D. Disease burden of chronic lymphocytic leukaemia within the European Union. Eur J Haematol. 2008;81(4):253–8.
Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–34.
Goede V, Fischer K, Engelke A, et al. Obinutuzumab as frontline of chronic lymphocytic leukemia: updated results of the CLL11 Study. Leukemia. 2015;29:1602–4.
INFARMED 2016. Reimbursement assessment report of obinutuzumab in CLL. Available from http://www.infarmed.pt/documents/15786/1424140/RelatorioPublico_Gazyvaro_obinutuzumab.pdf/bf56ae0f-aa28-4bb1-b3df-e5ac70562a06. Latest Access in 22-12-2016.
Eichhorst B, Dreyling M, Robak T, Monserrat E, Hallek M. Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi50–4.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Hodgkin’s Lymphomas. Version 2.2014. March 37, 2014. Latest Access in 11-07-2014.
Silva EA, Pinto CG, Sampaio C, Pereira JA, Drummond M e Trindade R, Orientações Metodológicas para Estudos de Avaliação Económica de Medicamentos, INFARMED; 1998.
Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Pharmacoeconomics. 2013;31:361–7.
Becker U, Briggs AH, Moreno S, et al. Cost-effectiveness model for chemoimmunotherapy options in patients with previously untreated chronic lymphocytic leukemia unsuitable for full-dose fludarabine-based therapy. Value Health. 2016;19(4):364–82.
Goede V, Fischer K, Busch R, et al. Obinutuzumab plus Chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10.
INE (Statistics Portugal) 2014, Portuguese general population mortality tables 2011–2013.
Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–91.
NICE. Guide to the methods of technology appraisal 2013. Available from http://publications.nice.org.uk/pmg9.
Kosmas CE, Shingler SL, Samanta K, et al. Health state utilities for chronic lymphocytic leukemia: importance of prolonging progression free survival. Leuk Lymphoma. 2015;56:1320–6.
Eichhorst B. Interview. Cologne: Department I of Internal Medicine, University Hospital of Cologne; 2014.
Ministério da Saúde, Diário da República, Nº 153 (Portaria nº 234/2015) de 7 de agosto de 2015.
ACSS, I.P. – Administração Central do Sistema de Saúde, Contabilidade Analítica 2006 – Hospitais do SNS, 2007. http://www2.acss.min-saude.pt/Portals/0/DownloadsPublicacoes/SNS/Info_gestao/Contab_Analitica_2006_Hospitais_SNS.pdf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Roche Farmacêutica Química, Lda. funded CISEP for the contribution of its members to the adaptation of the economic model to the Portuguese setting and subsequent development of this manuscript. ATP and LSM worked at CISEP during the development of this project. They are currently working at the Center for Evidence-Based Medicine (CEMBE, Faculty of Medicine/ULisboa). UB is an employee of F. Hoffmann-La Roche Ltd. CP is an employee of Roche Farmacêutica Química, Lda. CGP works at CISEP.
Author Contributions
ATP and LSM wrote the initial version of the manuscript. All authors commented on the initial version and its revisions and approved the final manuscript.
Appendices
Appendix 1: Akaike Information Criteria (AIC) for PFS, with ranks in brackets
Parametric distribution | GClb | RClb | Clb |
|---|---|---|---|
Exponential | 780.1 (6) | 808.8 (6) | 285.8 (6) |
Weibull | 744.4 (1) | 737.9 (4) | 268.2 (3) |
Log normal | 773.4 (5) | 715.3 (3) | 270.3 (4) |
Gamma | 746.3 (3) | 713.5 (2) | 265.0 (2) |
Log-logistic | 744.5 (2) | 697.5 (1) | 259.4 (1) |
Gompertz | 755.9 (4) | 782.9 (5) | 283.2 (5) |
Appendix 2: Expert panel results (resource consumption) and unit costs
2.1 Medicinal products and administration costs
Medicinal products | Unit cost (€) |
|---|---|
Obinutuzumab (1000 mg vial) | 3780.06a |
Rituximab (500 mg vial; 10 mg/mL) | 1023.88 |
Rituximab (100 mg vial; 10 mg/mL) | 209.49 |
Chlorambucil (25 tablets package) | 40.51 |
Administration | 175.24b |
2.2 Health state costs per month
Progression free (values per month) | Post-progression (values per month) | |||||||
|---|---|---|---|---|---|---|---|---|
Patients % | Number of times | Unit cost (€) | Weighed cost (€) | Patients % | Number of times | Unit cost (€) | Weighed cost (€) | |
Hospital services | ||||||||
Regular hospitalisation unit | 2.5 | 1 | 2654.94 | 66.37 | 20.0 | 1 | 5195.86 | 1039.17 |
Palliative care unit | 0.0 | 0 | – | – | 0.0 | 0 | – | – |
Outpatient services | ||||||||
Emergency episode | 7.5 | 1 | 82.99 | 6.22 | 22.5 | 1 | 82.99 | 18.67 |
Haematology/oncology visits | 30.0 | 1 | 31.00 | 9.30 | 100.0 | 1 | 31.00 | 31.00 |
General practice visits | 10.0 | 1 | 31.00 | 3.10 | 7.5 | 1 | 31.00 | 2.33 |
Pain visits | 2.5 | 1 | 51.60 | 1.29 | 2.5 | 1 | 51.60 | 1.29 |
Nutrition visits | 0.5 | 1 | 16.00 | 0.08 | 0.5 | 1 | 16.00 | 0.08 |
Nursery visits | 0.0 | 0 | 16.00 | – | 2.5 | 1 | 16.00 | 0.40 |
Psychiatry /psychology visits | 2.5 | 1 | 24.50 | 0.61 | 10.0 | 1 | 24.50 | 2.45 |
Other specialist visits | 50.0 | 1 | – | – | 50.0 | 1 | – | – |
Exams | ||||||||
Biochemistry examsa | 30.0 | 1 | 8.00 | 2.40 | 100.0 | 1 | 8.00 | 8.00 |
Complete blood count | 30.0 | 1 | 4.70 | 1.41 | 100.0 | 1 | 4.70 | 4.70 |
Glycaemia | 30.0 | 1 | 4.00 | 1.20 | 100.0 | 1 | 4.00 | 4.00 |
Chest x-ray | 10.0 | 1 | 9.00 | 0.90 | 30.0 | 1 | 9.00 | 2.70 |
CT scan | 2.5 | 1 | 73.30 | 1.83 | 10.0 | 1 | 73.30 | 7.33 |
Anatomopathology | 0.0 | 0 | 86.90 | – | 2.5 | 1 | 86.90 | 2.17 |
Myelogram | 0.0 | 0 | 26.50 | – | 5.0 | 1 | 26.50 | 1.33 |
FISH test | 0.0 | 0 | 287.50 | – | 8.3 | 1 | 287.50 | 23.86 |
Flow cytometry | 0.0 | 0 | 110.88 | – | 8.3 | 1 | 110.88 | 9.20 |
Concomitant therapy and outpatient procedures | ||||||||
G-CSF | 0.5 | 4 | 9.30 | 0.19 | 7.5 | 4 | 9.30 | 2.79 |
Immunoglobulins | 5.0 | 1 | 20 | 1.01 | 10.0 | 1 | 20.20 | 2.02 |
Blood transfusion | 0.5 | 1 | 125.10 | 0.63 | 5.0 | 1 | 125.10 | 6.26 |
Other therapies (ex. antibiotics, antidepressants, antihypertensives) | 100 | 0 | – | – | 100.0 | 0 | – | – |
Domiciliary care | 2.50 | 8696 | 33.10 | 7.20 | 2.5 | 8696 | 33.10 | 7.20 |
Total cost/month | 103.74 | Total cost/month | 1176.94 | |||||
2.3 Adverse event costs
Number | Patients % | Unit cost (€) | Weighed cost (€) | ||
|---|---|---|---|---|---|
Anaemia: grade 3 | |||||
Hospitalisation | 1 | 5 | 1282.84 | 64.14 | |
Emergency episode | 2 | 95 | 82.99 | 157.69 | |
Exams | Complete blood count | 2 | 95 | 4.70 | 8.93 |
Therapy | Blood transfusion | 1 | 95 | 125.10 | 118.85 |
Total cost per episode (€) | 349.60 | ||||
Neutropenia/leukopenia: grade 3/4 | |||||
Hospitalisation | 0 | 0 | – | – | |
Emergency episode | 1 | 100 | 82.99 | 82.99 | |
Outpatient visits | Haematology/oncology | 1 | 100 | 31.00 | 31.00 |
Exams | Complete blood count | 1 | 100 | 4.70 | 4.70 |
Biochemistry | 1 | 100 | 8.00 | 8.00 | |
PCR | 1 | 100 | 3.17 | 3.17 | |
Therapy | G-CSF | 4 | 65 | 9.30 | 24.18 |
Total cost per episode (€) | 154.04 | ||||
Thrombocytopenia: grade 3/4 | |||||
Hospitalisation | 1 | 2.5 | 2183.34 | 54.58 | |
Emergency episode | 1 | 98 | 82.99 | 80.92 | |
Outpatient visits | Haematology/oncology | 1 | 3 | 31.00 | 0.78 |
Exams | Complete blood count | 2 | 98 | 4.70 | 9.17 |
Biochemistry | 1 | 3 | 8.00 | 0.20 | |
Therapy | Platelet transfusion | 2 | 29 | 214.60 | 125.54 |
Total cost per episode (€) | 271.18 | ||||
Febrile neutropenia: grade 3/4 | |||||
Hospitalisation | 1 | 65 | 1282.84 | 833.85 | |
Emergency episode | 3 | 12 | 82.99 | 30.50 | |
1 | 23 | 82.99 | 18.88 | ||
Exams | Complete blood count | 2 | 30 | 4.70 | 1.73 |
Biochemistry | 2 | 30 | 8.00 | 2.94 | |
PCR | 2 | 30 | 3.17 | 1.16 | |
Blood culture | 1 | 12 | 12.00 | 1.47 | |
Urine culture | 1 | 12 | 10.50 | 1.29 | |
X-ray | 1 | 12 | 9.00 | 1.10 | |
CT scan | 1 | 4 | 73.30 | 2.57 | |
Therapy | Oral antibiotics | 1 | 12 | 3.02 | 0.37 |
G-CSF | 4 | 11 | 9.30 | 4.17 | |
Total cost per episode (€) | 903.63 | ||||
Infection/pneumonia: grade 3 | |||||
Hospitalisation | 1 | 100 | 1110.88 | 1110.88 | |
Total cost per episode (€) | 1110.88 | ||||
Other infections (urinary or respiratory): grade 3 | |||||
Hospitalisation | 1 | 90 | 1041.87 | 937.68 | |
Total cost per episode (€) | 937.68 | ||||
Injection site reaction: grade 3 | |||||
Hospitalisation | 1 | 2.5 | 1541.65 | 38.54 | |
Emergency episode | 1 | 98 | 82.99 | 80.92 | |
Exams | Biochemistry | 1 | 98 | 8.00 | 7.80 |
Complete blood count | 1 | 98 | 4.70 | 4.58 | |
Arterial blood gas analysis | 1 | 10 | 0.80 | 1.05 | |
Therapy | Antihistamines; corticoids; IV fluid therapy; oxygen; antipyretic | 1 | 98 | 11.24 | 10.96 |
Total cost per episode (€) | 143.86 | ||||
Maculopapular rash: grade 3 | |||||
Hospitalisation | 0 | 0 | – | – | |
Emergency episode | 1 | 100 | 82.99 | 82.99 | |
Outpatient visits | Haematology/oncology | 1 | 100 | 31.00 | 31.00 |
Dermatology | 1 | 100 | 31.00 | 31.00 | |
Therapy | Antihistamines and corticoids | 1 | 100 | 2.28 | 2.28 |
Total cost per episode (€) | 147.27 | ||||
Rights and permissions
About this article
Cite this article
Paquete, A.T., Miguel, L.S., Becker, U. et al. Cost‐Effectiveness Analysis of Obinutuzumab for Previously Untreated Chronic Lymphocytic Leukaemia in Portuguese Patients who are Unsuitable for Full-Dose Fludarabine-Based Therapy. Appl Health Econ Health Policy 15, 501–512 (2017). https://doi.org/10.1007/s40258-017-0321-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-017-0321-2