Abstract
Background
Paroxysmal supraventricular tachycardia (PSVT) treatment requires medically supervised intervention. Etripamil is a novel short-acting calcium channel blocker. Its intranasal spray formulation has a rapid onset of action and shows promise for the unsupervised treatment of PSVT.
Objective
We aimed to evaluate the efficacy and safety of etripamil nasal spray for the acute conversion of PSVT.
Methods
A systematic review and meta-analysis synthesizing randomized controlled trials (RCTs), which were retrieved by systematically searching the PubMed, EMBASE, Web of Science, SCOPUS, and Cochrane databases through to 1 December 2022. RevMan version 5.4 software was used to pool dichotomous outcomes using risk ratio (RR) presented with the corresponding confidence interval (CI).
Results
Three RCTs with a total of 496 participants were included in our analysis. Etripamil was effective for PSVT conversion at 15 min (RR 1.84, 95% CI 1.37–2.48), 30 min (RR 1.86, 95% CI 1.42–2.44), and 60 min (RR 1.25, 95% CI 1.05–1.50) after drug administration; decreasing medical intervention-seeking (RR 0.58, 95% CI 0.37–0.90); and decreasing emergency room (ER) visits (RR 0.61, 95% CI 0.38–0.97). However, there was no difference at 300 min (RR 1.10, 95% CI 0.97–1.25) and it was associated with higher rates of adverse events (RR 3.17, 95% CI 2.15–4.69).
Conclusion
Etripamil nasal spray was effective and well tolerated to induce PSVT termination for up to 60 min. Therefore, etripamil nasal spray constitutes a promising strategy for PSVT self-termination without medical supervision; however, further RCTs are required before endorsement in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A systematic review and meta-analysis synthesizing three randomized controlled trials, including 466 participants and investigating the efficacy and safety of etripamil (70 mg) versus placebo. |
Etripamil was effective for paroxysmal supraventricular tachycardia (PSVT) conversion up to 60 min, decreasing medical intervention-seeking and emergency department visits. |
Etripamil was also well tolerated, being associated with mild local adverse events such as nasal congestion and discomfort, without any serious adverse events or any cases of severe atrioventricular block. |
1 Introduction
Cardiac arrhythmias originating due to the automaticity of structures at or above the level of the atrioventricular (AV) node are defined as supraventricular tachycardias (SVTs). SVTs are temporary arrhythmias, including atrial flutter, atrial fibrillation, AV re-entrant tachycardia (AVRT) [Wolf-Parkinson-White syndrome], and AV nodal re-entrant tachycardia (AVNRT). The most common type of SVTs are AVNRTs, followed by AVRTs [1]. Patients with new-onset paroxysmal SVT (PSVT) or a history of PSVT have symptoms such as palpitations, dizziness, fainting, discomfort or pain in the chest, shortness of breath, anxiety, and nausea [2]. A 12-lead electrocardiography (ECG) reveals a narrow complex tachycardia during an episode of PSVT. These accelerated rhythms with sudden onset can cause significant patient morbidity. In the United States, after adjusting for age and sex, PSVT incidence was last reported at 36/100,000 persons/year [3]. PSVTs cause 50,000 admissions to the emergency room (ER) every year, costing $190 million [2].
The most performed initial treatment for PSVT are the vagal maneuvers, which result in the activation of the parasympathetic nervous system, slowing the heart rate. Another method involves massaging the carotid sinus; failure of these physical therapies is then followed by pharmacological therapies. Administration of AV node-blocking medications, such as the calcium channel blockers verapamil and diltiazem; antiarrhythmic medications that block the AV node and lengthen the AV refractory period, such as adenosine; or cardioversion in hemodynamically unstable patients. The onset of action for oral medications is longer compared with intravenous, intranasal, or sublingual medications [2].
Therefore, there is a need for a well-tolerated PSVT drug that can be self-administered via a quick onset of action. Etripamil is a novel, rapid-acting, L-type calcium channel blocker that is administered as a nasal spray. The compelling advantages of etripamil, including its intranasal delivery, rapid onset of action, and short half-life, make it a promising intervention for patients to self-administer outside of formal healthcare settings [2]. Etripamil has recently demonstrated encouraging safety and efficacy in recent randomized controlled trials (RCTs) [4,5,6]. Thus, we performed a systematic review and meta-analysis to investigate the efficacy and safety of etripamil nasal spray for the treatment of acute PSVT, and to review the current evidence about the possible role of etripamil in unsupervised PSVT management.
2 Methodology
This systematic review and meta-analysis was thoroughly conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [7] and the Cochrane Handbook of Systematic Reviews and Meta-Analysis [8].
2.1 Data Sources and Search Strategy
We conducted a comprehensive search of the following databases: PubMed, EMBASE, Web of Science, SCOPUS, and Cochrane Library through to 1 December 2022, without using any search limits. The comprehensive search terms and findings are elaborated in electronic supplementary Table S1.
2.2 Eligibility Criteria
We included RCTs with the following PICO criteria: population (P): patients with a history of PSVT; intervention (I): etripamil at a dose of 70 mg; control (C): placebo; outcome (O): efficacy outcomes of this review are conversion to sinus rhythm after 15, 30, 60, and 300 min, medical intervention-seeking, and ER visits. The safety outcomes include the incidence of any adverse event, any serious adverse event (SAE), nasal discomfort, nasal congestion, epistaxis, and rhinorrhea.
2.3 Study Selection
Two reviewers (SK and IG) independently screened the titles and abstracts of the articles identified in the search and assessed the full-text articles for eligibility based on predefined inclusion and exclusion criteria. Any disagreement was resolved through discussion or by a third reviewer (BA). The included studies were reported in a PRISMA flow diagram.
2.4 Data Extraction
Two independent investigators (SK and MAE) extracted the summary, baseline, and outcome data from the included RCTs, including study characteristics (country, study design, total participants, main inclusion criteria, the primary outcome, and follow-up duration); baseline characteristics (age, sex, number of patients in each group, mean PSVT confirmation duration, the mean number of PSVT episodes in the past year, lifetime number of emergency department [ED] visits for PSVT, and concomitant drug use); efficacy outcomes data (conversion to sinus rhythm after 15, 30, 60, and 300 min), medical intervention-seeking, and ER visits); and safety outcomes data (incidence of any adverse event, any SAE, nasal discomfort, nasal congestion, epistaxis, and rhinorrhea). Any disagreement was resolved through discussion or by a third reviewer (BA).
2.5 Risk of Bias and Quality Assessment
Two independent investigators (SK and MAE) implemented the Cochrane Collaboration's tool for assessing the risk of bias in RCTs (ROB 1) [9], considering selection, performance, detection bias, attrition, reporting, and other potential sources of biases. Any disagreement was resolved through discussion or by a third reviewer (BA). Furthermore, two independent investigators (BA and MA) used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to evaluate the quality of the evidence [10, 11].
2.6 Statistical Analysis
This meta-analysis was conducted using Revman software version 5.4 [12] to pool dichotomous outcomes using risk ratio (RR) along with the corresponding 95% confidence interval (CI). Pooled analysis was conducted using the fixed-effects model; however, the random-effects model was implemented in case of significant heterogeneity. Heterogeneity was evaluated using the Chi-square test and measured using the I-square test. The Chi-square test was considered significant at an alpha level below 0.1, and heterogeneity was considered significant if the I-square was > 50%. On significant heterogeneity, sensitivity analysis by excluding one study at a time and re-running the analysis was conducted to investigate the source of heterogeneity. Finally, we did not investigate the publication bias by funnel plots as we included fewer than 10 RCTs [13].
3 Results
3.1 Search Results and Study Selection
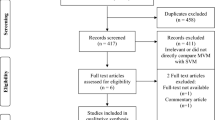
We retrieved 57 records after searching the databases and 35 duplicates were excluded using Covidence, leaving 22 records to be screened. After title and abstract screening, we proceeded with 16 articles for full-text screening. Finally, we included three RCTs [4,5,6]; two through searching databases [4, 5], and another was added manually from a 2022 American Heart Association (AHA) Scientific Sessions conference presentation [6] (Fig. 1).
3.2 Characteristics of the Included Studies
The final analysis included three RCTs [4,5,6], with a total of 496 patients: 296 in the etripamil group and 200 in the placebo group. The NODE-1 trial is a multicenter, double-blinded, dose-ranging, phase II RCT [5], and NODE-301 [4] and RAPID [6] are multicenter, double-blinded, phase III RCTs. Only RAPID allowed second dose administration after 10 min in cases of non-response to the first dose, while NODE-1 and NODE-301 administrated a single dose. Furthermore, we only included 23 participants from NODE-1 who were administered etripamil 70 mg. The detailed summary characteristics of the included RCTs and baseline data of the participants are outlined in Tables 1 and 2.
3.3 Risk of Bias and Quality of Evidence
The included RCTs showed a low risk of bias in selection, performance, attrition, and reporting biases. However, NODE-1 showed an unclear risk of detection bias and all three RCTs showed a high risk of other biases, being funded by the manufacturer’s pharmaceutical company (Milestone Pharmaceuticals) (Fig. 2). The quality of evidence assessment is outlined in Table 3.
Quality assessment of the risk of bias in the included trials. The upper panel presents a schematic representation of risks (low = red, unclear = yellow, and high = red) for specific types of biases of each of the studies included in the review. The lower panel presents risks (low = red, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review
3.4 Efficacy Outcomes
Etripamil was effective for PSVT conversion after 15 min (RR 1.84, 95% CI 1.37–2.48; p = 0.0001) [low-quality evidence], 30 min (RR 1.86, 95% CI 1.42–2.44; p = 0.00001) [low-quality evidence], and 60 min (RR 1.25, 95% CI 1.05–1.50; p = 0.01) [very low-quality evidence] after drug administration; decreasing medical intervention-seeking (RR 0.58, 95% CI 0.37–0.90; p = 0.01) [very low-quality evidence]; and decreasing ER visits (RR 0.61, 95% CI 0.38–0.97; p = 0.04) [very low-quality evidence]. However, there was no difference at 300 min (RR 1.10, 95% CI 0.97–1.25; p = 0.12) [very low-quality evidence] (Fig. 3, Table 3).
Studies were homogenous in PSVT conversion after 15 min (p = 0.56, I2 = 0%), 30 min (p = 0.28, I2 = 14%), 60 min (p = 0.27, I2 = 17%), and 300 min after drug administration (p = 0.29, I2 = 11%); decreasing medical intervention-seeking (p = 0.74, I2 = 0%); and decreasing ER visits (p = 0.64, I2 = 0%).
3.5 Safety Outcomes
Etripamil was associated with higher rates of the incidence of any AEs (RR 3.17, 95% CI 2.15–4.69; p = 0.00001) [low-quality evidence], nasal discomfort (RR 3.82, 95% CI 2.01–7.24; p = 0.0001) [low-quality evidence], nasal congestion (RR 5.89, 95% CI 1.93–17.97; p = 0.002) [low-quality evidence], epistaxis (RR 4.74, 95% CI 1.21–18.50; p = 0.03) [very low-quality evidence], and rhinorrhea (RR 3.53, 95% CI 1.22–10.26; p = 0.02) [very low-quality evidence], with no difference regarding SAE incidence (RR 0.30, 95% CI 0.01–7.21; p = 0.46) [very low-quality evidence] (Fig. 4, Table 3).
Studies were homogenous in the incidence of any AEs (p = 0.61, I2 = 0%), nasal discomfort (p = 0.50, I2 = 0%), epistaxis (p = 0.59, I2 = 0%), and rhinorrhea (p = 0.99, I2 = 0%); however, nasal congestion showed mild heterogeneity (p = 0.13, I2 = 56%).
4 Discussion
Our analysis revealed that etripamil is effective to terminate SVT, converting it to sinus rhythm at 15, 30, and 60 min, decreasing medical intervention-seeking and visits to the ER. However, etripamil has been associated with some adverse events, including nasal discomfort, congestion, epistaxis, and rhinorrhea.
Etripamil is a short-acting calcium channel blocker but its exact pharmacokinetics and pharmacodynamics are yet to be determined. Nevertheless, nasal delivery of drugs improves bioavailability by avoiding first-pass metabolism in the liver, and reduces slow absorption, drug degradation, and gastrointestinal adverse effects [14]. PSVT has a high recurrence rate; hence, treatment with early onset and a long therapeutic effect is necessary, and this is compatible with etripamil’s pharmacokinetic features. Regarding conversion to sinus rhythm, etripamil has variable response rates due to its half-life, which may explain why it can only be detected for up to 300 min. Conversely, adenosine, which blocks calcium influx into cardiac cells, is an effective therapy (86–96%) for the rapid termination of PSVT [15]. However, due to its short half-life (10–30 s), its effect is short-lived and the SVT might recur. However, the half-life of etripamil is around 20 min, which can effectively prevent PSVT recurrence [2]. Thus, Stambler et al. [4] argue that a time point of 30 min is the most appropriate to estimate the half-life of etripamil, suiting its pharmacokinetic properties. Furthermore, follow-up with patients for up to 5 h was conducted to detect any efficacy of etripamil, as NODE-301 and RAPID were conducted outside clinical settings [4, 6]. Moreover, different response rates may be associated with pharmacogenetics, and more research is needed to investigate how genetic differences affect how well a treatment works.
SVT is a common cause of hospital admissions with regard to medical intervention-seeking and ER visits. The incidence of PSVT is about 36/100,000 persons, with a female predominance [15, 16]. As per the editorial by Naccarelli et al. [17], the recurrence of PSVT depends on the frequency of exposure to triggers, the properties of the PSVT-involved pathways, and the amount of their excitable gap. Etripamil could be a potential alternative for aborting PSVT in outpatient settings due to its ease of self-administration and effective response. Furthermore, the availability of outpatient intranasal etripamil may significantly decrease patients’ exposure to inappropriate or excess therapy and unnecessary investigations and procedures, which is supported by our analysis. The study conducted by O’Rourke et al. [18] discovered that 38% of patients in the ED were administered adenosine for sinus, ventricular tachycardia, and atrial fibrillation. Moreover, etripamil can be administered as an adjuvant to oral medications before catheter ablation in case of any delay in getting to the procedure.
Etripamil seems to be a safer, easier, and more effective treatment option than oral or intravenous medications. Vagal maneuvers are recommended as the first line of treatment for acute SVT [2]. The Valsalva maneuver, a type of vagal maneuver, involves patient expiration against a closed glottis to stimulate the vagal nerve [19]. However, the efficacy of the Valsalva maneuver varies widely, from 19.4 to 54.3% [19]. The REVERT trial showed that the modified Valsalva technique, which combines passive leg lifting with supine posture, has higher conversion rates compared with the standard Valsalva technique (43% vs. 17%) [19]. However, this modified technique requires a healthcare practitioner and the conversion rate is not as significant as with etripamil. Other vagal maneuvers involve carotid sinus massaging, which is effective but can lead to stroke in the elderly with a history of cardiovascular disease as it carries a risk of embolization [19]. If the above-mentioned maneuvers fail, the first medication administered is adenosine, which is nearly 90% effective with a rapid onset and a very short half-life [2]. However, adenosine is administered intravenously, requiring hospitalization [20]. Furthermore, intravenous calcium channel blockers, such as verapamil and diltiazem, used in hemodynamically stable SVT patients are associated with an excess decrease in heart rate and contractility [20].
Moreover, etripamil was associated with a small number of mild local adverse events, including nasal congestion, nasal discomfort, rhinorrhea, and epistaxis. There was no evidence of QT interval prolongation or AV block. Only one patient experienced hypotension with type II second-degree AV block, 5 min after receiving etripamil 140 mg in NODE-1 [5], which was avoided by using a lower dosage (70 mg) in NODE-301 and RAPID without a single case of second- or third-degree AV block [4, 6]. Conversely, current intravenous calcium channel blockers and β-blockers have been frequently associated with AV block [21]. Accordingly, etripamil is well tolerated and effective for PSVT conversion without medical supervision.
4.1 Strengths and Limitations
To the best of our knowledge, this is the first systematic review and meta-analysis to assess the safety and efficacy of etripamil for PSVT termination. We compiled each study’s findings, limitations, and conclusions into a thorough quantitative and qualitative analysis following Cochrane [8], GRADE [10, 11], and PRISMA guidelines [7]. In addition, we presented the results of various RCTs, which included the administration of etripamil in clinical and unsupervised settings. Therefore, our study constitutes the gold-standard evidence in this regard.
However, our review has some limitations. First, our analysis is limited by the small number of included RCTs, and NODE-1 included patients under sedation and artificially induced PSVT [5], compared with the alert patients with spontaneous PSVT in NODE-301 and RAPID [4, 6]. Second, significant variations in baseline characteristics, such as the mean number of PSVT episodes in the past year, being higher in the placebo group in both NODE-301 and RAPID, can affect our findings. Furthermore, the age of patients included in the studies was wide (> 18 years); however, the mean age of the included patients was in the 50s, indicating that young patients were underrepresented in the results. Third, we could not do a subgroup analysis for different PSVT subtypes such as AVNRT and AVRT because of the lack of data. Fourth, individuals who were taking certain medications and had structural heart disease were excluded from the trials. This might impede the treatment’s use by a significant number of patients. Finally, all our included outcomes showed low- to very low-quality evidence, which limits the generalizability of our findings.
4.2 Implications for Future Research
Further studies are required to study the long-term safety of etripamil and its efficacy in special populations, such as pediatric subjects. Etripamil may also be beneficial in the treatment of other conditions that respond to calcium channel blockers, such as atrial fibrillation and angina; hence, investigating this can be beneficial. Given the high cost of ED care, antiarrhythmic drugs, and successful ablation, this approach will be significantly less expensive. However, pharmacoeconomic studies are also required.
Moreover, further studies are required to examine the safety of etripamil among patients with pre-excitation in baseline ECG.
5 Conclusion
Etripamil is a promising candidate for self-termination of symptomatic, sustained PSVT in a non-clinical setting, with an acceptable safety and efficacy profile. Large-scale, double-blind RCTs with long follow-up periods are needed to confirm our findings before etripamil clinical endorsement.
References
Colucci RA, Silver MJ, Shubrook J. Common types of supraventricular tachycardia: diagnosis and management. Am Fam Phys. 2010;82:942–52.
Chu GS, Gupta D. Update on etripamil nasal spray for the at-home treatment of acute paroxysmal supraventricular tachycardia. Heart Int. 2021;15:2–6.
Orejarena LA, Vidaillet H, Destefano F, Nordstrom DL, Vierkant RA, Smith PN, et al. Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol. 1998;31:150–7.
Stambler BS, Plat F, Sager PT, Shardonofsky S, Wight D, Potvin D, et al. First randomized, multicenter, placebo-controlled study of self-administered intranasal etripamil for acute conversion of spontaneous paroxysmal supraventricular tachycardia (NODE-301). Circ Arrhythmia Electrophysiol. 2022;15:E010915.
Stambler BS, Dorian P, Sager PT, Wight D, Douville P, Potvin D, et al. Etripamil nasal spray for rapid conversion of supraventricular tachycardia to sinus rhythm. J Am Coll Cardiol. 2018;72:489–97.
Stambler BS, Camm AJ, Alings M, Dorian P, Heidbuchel H, Houtgraaf J, et al. Self-administered etripamil for termination of spontaneous paroxysmal supraventricular tachycardia: primary analysis from the rapid study. In: Presented at the American Heart Association Scientific Sessions. 2022.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;2021:372. https://www.bmj.com/content/372/bmj.n71. Accessed 13 Aug 2021.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. In: Cochrane handbook of systematic reviews of interventions. 2019; pp. 1–694.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;2011:343. https://www.bmj.com/content/343/bmj.d5928. Accessed 22 Jul 2021.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. Rating quality of evidence and strength of recommendations: what is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995. /labs/pmc/articles/PMC2364804/. Accessed 23 Dec 2021.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Rating Quality of Evidence and Strength of Recommendations: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924. /labs/pmc/articles/PMC2335261/. Accessed 23 Dec 2021.
Twells LK. Evidence-based decision-making 1: critical appraisal. Methods Mol Biol. 2015;1281:385–96. http://www.jamaevidence.com. Accessed 26 May 2021.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ; 1997;315:629–34. https://www.bmj.com/content/315/7109/629. Accessed 3 Aug 2021.
Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013;3:42–62.
Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67:e27-115.
Brugada J, Demosthenes GK, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:655–720.
Naccarelli GV, Wolbrette DL, Gonzalez MD. Can an intranasal calcium-channel blocker convert paroxysmal supraventricular tachycardia and keep the doctor away? J Am Coll Cardiol. 2018;72:498–500.
O’Rourke SF, Sauvage A, Evans PA. Paroxysmal supraventricular tachycardia: improving diagnosis and management within the accident and emergency department. Emerg Med J. 2004;21:495–7.
Smith G. Management of supraventricular tachycardia using the Valsalva manoeuvre: a historical review and summary of published evidence. Eur J Emerg Med. 2012;19:346–52.
DiMarco JP, Sellers TD, Berne RM, West GA, Belardinelli L. Adenosine: electrophysiologic effects and therapeutic use for terminating paroxysmal supraventricular tachycardia. Circulation. 1983;68:1254–63.
Lim SH, Anantharaman V, Teo WS, Chan YH. Slow infusion of calcium channel blockers compared with intravenous adenosine in the emergency treatment of supraventricular tachycardia. Resuscitation. 2009;80:523–8.
Acknowledgments
None.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
MA and BA conceived the idea; BA and MA designed the research workflow; BA and MA searched the databases; SK, IG, and MAE screened the retrieved records, extracted relevant data, and assessed the quality of evidence, and BA resolved any conflicts; MA performed the analysis; KA, MA, and OS wrote the final manuscript, and BA supervised the project. All authors have read and agreed to the final version of the manuscript
Conflicts of interest
Mohamed Abuelazm, Soumya Kambalapalli, Othman Saleh, Mohamed A. Elzeftawy, Khaled Albakri, Ibrahim Gowaily, and Basel Abdelazeem declare no conflicts of interest.
Funding
No funding was received to assist in the preparation of this study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Abuelazm, M., Kambalapalli, S., Saleh, O. et al. The Efficacy and Safety of Etripamil Nasal Spray for Acute Paroxysmal Supraventricular Tachycardia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Cardiovasc Drugs 23, 379–391 (2023). https://doi.org/10.1007/s40256-023-00592-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-023-00592-7