Abstract
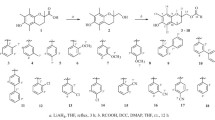
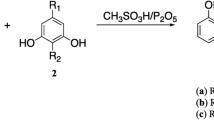
In this study, esculetin(1) was chosen as a lead compound and some structural modifications were designed to explore the antioxidant activities of esculetin derivatives. Meanwhile, a convenient method for selective methylation of catechol coumarins with different bases was developed. Furthermore, a few 5,7-dihydroxylcoumarins were synthesized and 7-hydroxylcoumarins were employed in order to explore the potential structure-antioxidant activity relationships. The antioxidant activities of these compounds were evaluated and compared with standard antioxidant Trolox by the 2,2′-diphenyl-1-picrylhydrazyl(DPPH) assay, 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonate) cation(ABTS+) assay and ferric reducing antioxidant power(FRAP) assay. The results show that the catechol group is the key pharmacophore. Meanwhile, introducing electronegative groups at the C4 position of esculetin(1) may enhance the antioxidative capacity, while introducing a group containing nitrogen as a hydrogen bond acceptor at the C8 position may slightly reduce the antioxidative capacity. Among them, the most powerful antioxidants are com-pounds 5 and 7, which exhibit higher antioxidant activity than esculetin(1) in all assays.
Similar content being viewed by others
References
Hoelzl C., Bichler J., Ferk F., Simic T., Nersesyan A., Elbling L., Ehrlich V., Chakraborty A., Knasmüller S., J. Physiol. Pharmacol., 2005, 56, 49
Zhang Y., Zou B., Chen Z., Pan Y., Wang H., Liang H., Yi X., Bioorg. Med. Chem. Lett., 2011, 21, 6811
Borges F., Roleira F., Milhazes N., Santana L., Uriarte E., Curr. Med. Chem., 2005, 12, 887
Wu L., Wang X., Xu W., Farzaneh F., Xu R., Curr. Med. Chem., 2009, 16, 4236
Riveiro M. E., de Kimpe N., Moglioni A., Vazquez R., Monczor F., Shayo C., Davio C., Curr. Med. Chem., 2010, 17(13), 1325
Matos M. J., Santana L., Uriarte E., Delogu G., Corda M., Fadda M. B., Era B., Fais A., Bioorg. Med. Chem. Lett., 2011, 21, 3342
Galkin A., Fallarero A., Vuorela P. M., J. Pharm. Pharmacol., 2009, 61(2), 177
Panteleon V., Kostakis I. K., Marakos P., Pouli N., Andreadou I., Bioorg. Med. Chem. Lett., 2008, 18, 5781
Symeonidis T., Chamilos M., Hadjipavlou-Litina D. J., Kallitsakis M., Litinas K. E., Bioorg. Med. Chem. Lett., 2009, 19, 1139
Roussaki M., Kontogiorgis C. A., Hadjipavlou-Litina D., Hamilakis S., Detsi A., Bioorg. Med. Chem. Lett., 2010, 20, 3889
Liu Z. H., Wang Y. N., Sun J. B., Yang Y., Liu Q. W., Liu Z. Q., Song Z. G., Chem. Res. Chinese Universities, 2015, 31(4), 526
Masamoto Y., Ando H., Murata Y., Shimoishi Y., Tada M., Takahata K., Biosci. Biotechnol. Biochem., 2003, 67, 631
Lin H. C., Tsai S. H., Chen C. S., Chang Y. C., Lee C. M., Lai Z. Y., Lin C. M., Biochem. Pharmacol., 2008, 75(6), 1416
Potapovich M. V., Metelitza D. I., Shadyro O. I., Appl. Biochem. Mi-cro., 2012, 48(3), 250
Wang P., Xia Y. L., Yu Y., Lu J. X., Zou L. W., Feng L., Ge G. B., Yang L., RSC Adv., 2015, 5, 53477
Cao J. L., Shen S. L., Yang P., Qu J., Org. lett., 2013, 15(15), 3856
Lu J. X., Wang P., Hou J., Zou L. W., Cui P., Yang L., Ge G. B., Gong X. J., Chem. Res. Chinese Universities, 2016, 32(5), 786
Shahidi F., Liyana-Pathirana C. M., Wall D. S., Food Chem., 2006, 99, 478
Ozgen M., Reese R. N., Tulio J. A. Z., Scheerens J. C., Miller A. R., J. Agric. Food Chem., 2006, 54, 1151
Benzie I. F. F., Strain J., J. Anal. Biochem., 1996, 239, 70
Koleva I. I., van Breek T. A., Linssen J. P. H., Groot A. D., Evstatieva L. N., Phytochem. Anal., 2002, 13, 8
Lu Y., Huang J., Li Y., Ma T., Sang P., Wang W., Gao C., Food Chem., 2015, 183, 91
Firuzi O., Lacanna A., Petrucci R., Marrosu G., Saso L., Biochim. Biophys. Acta, 2005, 1721, 174
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Basic Research Program of China(No.2013CB531800), the National Natural Science Foundation of China(Nos.81402822, 81603187, 31471923, 31601517) and the Fundamental Research Funds for the Central Universities of China(No.DC201501020101).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chen, C., Wang, P., Zou, L. et al. Synthesis and biological evaluation of hydroxylcoumarin derivatives as antioxidant agents. Chem. Res. Chin. Univ. 33, 194–199 (2017). https://doi.org/10.1007/s40242-017-6411-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-017-6411-8