Abstract
Objectives
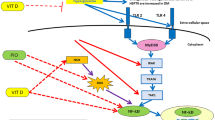
Diabetes mellitus (DM) is an important public health problem all over the world, considering its complications and increasing prevalence. Oleanolic acid (OA) has anti-diabetic property via modulating glucose metabolism and acting as 5′–adenosine monophosphate (AMP)–activated protein kinase (AMPK) / Sirtuin–1 (SIRT–1) activator and Interleukin 6 (IL–6) / Nuclear factor kappa B (NF–κB) inhibitor. This research questioned if the OA treatment amliorates the hepatic inflammatory profile in the diabetic rats.
Methods
Twenty–eight male Sprague Dawley rats were first subjected to either no diabetes induction (healthy) or diabetes induction by i.p. injection of 50 mg/kg streptozotocin. Then rats in both groups were treated with either tap water or OA (5 mg/kg) within 1 ml tap water by oral gavage for 21 days.
Results
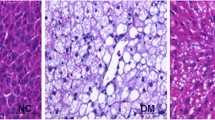
The diabetic rats had higher hepatic MDA (2.88x) and serum AST (2.01x), ALP (2.22x), and ALT (4.27x) levels and 50% lower hepatic SOD level than the healthy rats. The OA treatment significantly reversed these antioxidant parameters in the diabetic rats. The diabetic rats had lower AMPK (85%) and hepatic SIRT–1 (47%) levels and higher hepatic NF–κB (53%) and IL–6 (34%) levels than the healthy rats. Comparing with the health rats, the OA treatment increased hepatic SIRT–1 level, but tended to increase hepatic AMPK level and decrease hepatic NF–κB and IL–6 levels in the diabetic rats. It was also partially effective to ameliorate degenerative changes and necrosis in the diabetic rats.
Conclusion
The OA treatment can be considered to alleviate oxidative stress and reduce severity of inflammation in hepatocytes in the diabetic subjects.

Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine amino transferase
- AST:
-
Aspartate amino transferase
- ALP:
-
Alkaline phosphatase
- AMPK:
-
5′-adenosine monophosphate (AMP)-activated protein kinase
- DM:
-
Diabetes mellitus
- H&E:
-
Haematoxylin-Eosin
- IDF:
-
International Diabetes Federation
- IL-6:
-
Interleukin-6
- MDA:
-
Malondialdehyde
- NF-κB:
-
Nuclear factor kappa B
- OA:
-
Oleanolic acid
- ROS:
-
Reactive oxygen species
- SIRT-1:
-
Sirtuin-1
- STZ:
-
Streptozotocin
- SOD:
-
Superoxide dismutase
References
Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep. 2019;21(4):21. https://doi.org/10.1007/s11886-019-1107-y.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. https://doi.org/10.1016/j.diabres.2019.107843.
Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–90. https://doi.org/10.1038/s41581-020-0278-5.
Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43(1):51–64. https://doi.org/10.1111/j.1872-034X.2012.01031.x.
Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251. https://doi.org/10.1038/nrm3311.
Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48(4):e224. https://doi.org/10.1038/emm.2016.16.
Kişmiroğlu C, Cengiz S, Yaman M. Biochemistry of AMPK: mechanisms of action and importance in the treatment of diabetes. Eur J Sci Techn. 2020;18:162–70. https://doi.org/10.31590/ejosat.676335.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–8. https://doi.org/10.1038/nature03354.
Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280(21):20589–95. https://doi.org/10.1074/jbc.M412357200.
Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab J. 2013;37(5):315–25. https://doi.org/10.4093/dmj.2013.37.5.315.
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298(4):E751-60. https://doi.org/10.1152/ajpendo.00745.2009.
Jiang X, Chen J, Zhang C, Zhang Z, Tan Y, Feng W, et al. The protective effect of FGF21 on diabetes-induced male germ cell apoptosis is associated with up-regulated testicular AKT and AMPK/Sirt1/PGC-1α signaling. Endocrinology. 2015;156(3):1156–70. https://doi.org/10.1210/en.2014-1619.
Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26(3):685–98. https://doi.org/10.1007/s10787-018-0458-0.
Iskender H, Yenice G, Dokumacioglu E, Hayirli A, Sevim C, Dokumacioglu A, et al. Astaxanthin alleviates renal damage of rats on high fructose diet through modulating NFκB/SIRT1 pathway and mitigating oxidative stress. Arch Physiol Biochem. 2020;126(1):89–93. https://doi.org/10.1080/13813455.2018.1493609.
Mitchell JP, Carmody RJ. NF-κB and the Transcriptional control of inflammation. Int Rev Cell Mol Biol. 2018;335:41–84. https://doi.org/10.1016/bs.ircmb.2017.07.007.
Kunnumakkara AB, Shabnam B, Girisa S, Harsha C, Banik K, Devi TB, et al. Inflammation, NF-kappaB, and chronic diseases: how are they linked? Crit Rev Immunol. 2020;40(1):1–39. https://doi.org/10.1615/CritRevImmunol.2020033210.
Huang G, Mei X, Hu J. The antioxidant activities of natural polysaccharides. Curr Drug Targets. 2017;18(11):1296–300. https://doi.org/10.2174/1389450118666170123145357.
Yu Z, Sun W, Peng W, Yu R, Li E, Jiang T, et al. Pharmacokinetics in vitro and in vivo of two novel prodrugs of oleanolic acid in rats and its hepatoprotective effects against liver injury induced by CCl4. Mol Pharmacol. 2016;13(5):1699–710. https://doi.org/10.1021/acs.molpharmaceut.6b00129.
Ayeleso TB, Matumba MG, Mukwevho E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules. 2017;22(11):1915. https://doi.org/10.3390/molecules22111915.
Sen A. Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J Clin Cases. 2020;8(10):1767–92. https://doi.org/10.12998/wjcc.v8.i10.1767.
Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456–80. https://doi.org/10.4239/wjd.v6.i3.456.
Mohamed J, Nafizah AHN, Zariyantey AH, Budin SB. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16(2):e132-41. https://doi.org/10.18295/squmj.2016.16.02.002.
Dokumacioglu E, Iskender H, Sen TM, Ince I, Dokumacioglu A, Kanbay Y, et al. The effects of hesperidin and quercetin on serum tumor necrosis factor–alpha and interleukin–6 levels in streptozotocin–induced diabetes model. Pharmacogn Mag. 2018;14:167–173. https://doi.org/10.18295/squmj.2016.16.02.002.
Iskender H, Dokumacioglu E, Terim-Kapakin KA, Yenice G, Mohtare B, Bolat I, et al. Effects of oleanolic acid on inflammation and metabolism in diabetic rats. Biotech Histochem. 2021;15:1–8. https://doi.org/10.1080/10520295.2021.1954691.
Zhao H, Liu J, Song L, Liu Z, Han G, Yuan D, et al. Oleanolic acid rejuvenates testicular function through attenuating germ cell DNA damage and apoptosis via deactivation of NF–κB, p53 and p38 signaling pathways. J Pharm Pharmacol. 2017;69(3):295–304. https://doi.org/10.1111/jphp.12668.
Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hidroxynonenal. Methods Enzymol. 1990;186:407–21. https://doi.org/10.1016/0076-6879(90)86134-h.
Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. https://doi.org/10.1093/clinchem/34.3.497.
Terim–Kapakin KA, Gumus R, Imik H, Kapakin S, Saglam YS. Effects of ascorbic and α–lipoic acid on secretion of HSP–70 and apoptosis in liver and kidneys of broilers exposed to heat stress. Ankara Univ Vet J. 2012;59:279–87. https://doi.org/10.1501/Vetfak_0000002539.
Apaydin-Yildirim B, Kordali S, Terim-Kapakin KA, Yildirim F, Aktas-Senocak E, Altın S. Effect of Helichrysum plicatum DC. Subsp, plicatum ethanol extract on gentamicin-induced nephrotoxicity in rats. J Zhejiang Univ Sci B. 2017;18(6):501–11. https://doi.org/10.1631/jzus.B1500291.
Jugran AK, Rawat S, Devkota PH, Bhatt ID, Rawal RS. Diabetes and plant-derived natural products: from ethnopharmacological approaches to their potential for modern drug discovery and development. Phytother Res. 2021;35(1):223–45. https://doi.org/10.1002/ptr.6821.
Hedayat KM, Lapraz JC, Schuff B. Medicinal plants in clinical practice. The theory of endobiogeny 5th edition 2020;57–60
Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician. 2016;8(1):1832–42. https://doi.org/10.19082/1832.
Salleh NH, Zulkipli IN, Mohd YH, Ja’afar F, Ahmad N, Wan WA, et al. Systematic review of medicinal plants used for treatment of diabetes in human clinical trials: An ASEAN perspective. Evid Based Complement Alternat Med. 2021;2021:5570939. https://doi.org/10.1155/2021/5570939.
Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact. 2010;186(2):200–10. https://doi.org/10.1016/j.cbi.2010.03.028.
Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. https://doi.org/10.1155/2014/360438.
Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of oxidative stress during diabetes mellitus. J Biomark. 2013;2013:378790. https://doi.org/10.1155/2013/378790.
Masola B, Oguntibeju OO, Oyenihi AB. Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed Pharmacother. 2018;101:447–57. https://doi.org/10.1016/j.biopha.2018.02.115.
Saha MR, Dey P, Sarkar IDe Sarker D, et al. Acacia nilotica leaf improves insulin resistance and hyperglycemia associated acute hepatic injury and nephrotoxicity by improving systemic antioxidant status in diabetic mice. J Ethnopharmacol. 2018;210:275–86. https://doi.org/10.1016/j.jep.2017.08.036.
Anwer T, Alkarbi ZA, Hassan NA, Alshahrani S, Khan G, et al. Modulatory effect of zingerone against STZ-nicotinamide induced type-2 diabetes mellitus in rats. Arch Physiol Biochem. 2021;127(4):304–10. https://doi.org/10.1080/13813455.2019.1637436.
Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang X, Cui XG, et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med. 2020;24(21):12355–67. https://doi.org/10.1111/jcmm.15725.
Wang N, Zhang J, Qin M, Yi W, Yu S, Chen Y, et al. Amelioration of streptozotocin induced pancreatic β cell damage by morin: involvement of the AMPK FOXO3 catalase signaling pathway. Int J Mol Med. 2018;41(3):1409–18. https://doi.org/10.3892/ijmm.2017.3357.
Wang X, Li YL, Wu H, Liu JZ, Hu JX, Liao N, et al. Antidiabetic effect of oleanolic acid: a promising use of a traditional pharmacological agent. Phytother Res. 2011;25(7):1031–40. https://doi.org/10.1002/ptr.3385.
Hasanvand A, Amini-Khoei H, Hadian MR, Abdollahi A, Tavangar SM, Dehpour AR, et al. Anti-inflammatory effect of AMPK signaling pathway in rat model of diabetic neuropathy. Inflammopharmacology. 2016;24(5):207–19. https://doi.org/10.1007/s10787-016-0275-2.
Ban Q, Cheng J, Sun X, Jiang Y, Guo M. Effect of feeding type 2 diabetes mellitus rats with synbiotic yogurt sweetened with monk fruit extract on serum lipid levels and hepatic AMPK (5′ adenosine monophosphate-activated protein kinase) signaling pathway. Food Funct. 2020;11(9):7696–706. https://doi.org/10.1039/d0fo01860k.
Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48(7):e245-5. https://doi.org/10.1038/emm.2016.81.
Tanyıldız S, Yıldırım H, Uğur H, Yaman M. AMPK’s natural activators and relationships with diseases. Eur J Sci Technol. 2021;21:389–401. https://doi.org/10.31590/ejosat.762959.
Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage α1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285(25):19051–9. https://doi.org/10.1074/jbc.M110.123620.
Abedimanesh N, Asghari S, Mohammadnejad K, Daneshvar Z, Rahmani S, Shokoohi S, et al. The antidiabetic effects of betanin in streptozotocininduced diabetic rats through modulating AMPK/SIRT1/NFκB signaling pathway. Nutr Metab (Lond). 2021;18(1):92. https://doi.org/10.1186/s12986-021-00621-9.
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Sign. 2013;25(10):1939–48. https://doi.org/10.1016/j.cellsig.2013.06.007.
Gregorio E, Colell A, Morales A, Marí M. Relevance of SIRT1-NF-kappaB Axis as therapeutic target to ameliorate inflammation in liver disease. Int J Mol Sci. 2020;21(11):3858. https://doi.org/10.3390/ijms21113858.
Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS ONE. 2012;7(9):e46364. https://doi.org/10.1371/journal.pone.0046364.
Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:1607. https://doi.org/10.3389/fphys.2019.01607.
Manna P, Das J, Ghosh J, Sil PC. Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IkappaBalpha/NF-kappaB, MAPKs, and mitochondria-dependent pathways: prophylactic role of arjunolic acid. Free Radic Biol Med. 2010;48(11):1465–84. https://doi.org/10.1016/j.freeradbiomed.2010.02.025.
Romagnoli M, Gomez-Cabrera MC, Perrelli MG, Biasi F, Pallardó FV, Sastre J, et al. Xanthine oxidase-induced oxidative stress causes activation of NF-kappaB and inflammation in the liver of type I diabetic rats. Free Radic Biol Med. 2010;49(2):n171-7. https://doi.org/10.1016/j.freeradbiomed.2010.03.024.
Svistounov D, Smedsrød B. Hepatic clearance of advanced glycation end products (AGEs): myth or truth? J Hepatol. 2004;41:1038–40. https://doi.org/10.1016/j.jhep.2004.10.004.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. https://doi.org/10.1001/jama.286.3.327.
Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. In vitro TNF-alpha and IL-6 production by adherent peripheral blood mononuclear cells obtained from type 1 and type 2 diabetic patients evaluated according to the metabolic control. Ann NY Acad Sci. 2006;1079:177–80. https://doi.org/10.1196/annals.1375.027.
Pollier J, Goossens A, Oleanolic acid. Phytochemistry. 2012;77:10–5. https://doi.org/10.1016/j.phytochem.2011.12.022.
Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75(15):1549–60. https://doi.org/10.1055/s-0029-1186102.
An Q, Hu Q, Wang B, Cui W, Wu F, Ding Y. Oleanolic acid alleviates diabetic rat carotid artery injury through the inhibition of NLRP3 inflammasome signaling pathways. Mol Med Rep. 2017;16(6):8413–9. https://doi.org/10.3892/mmr.2017.7594.
Camer D, Yu Y, Szabo A, Huang XF. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol Nutr Food Res. 2014;58:1750–9. https://doi.org/10.1002/mnfr.201300861.
Yaman T, Uyar A, Celik I, Alkan EE, Keles OF, Yener Z. Histopathological and immunohistochemical study of antidiabetic effects of heracleum persicum extract in experimentally diabetic rats. IJPER. 2017;51(3):450–7. https://doi.org/10.5530/ijper.51.3s.66.
Funding
This work was supported by Coordinator of Scientific Research Projects [2021.M84.02.04] at Artvin Coruh University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was carried out in the Atatürk University’s Experimental Animal Laboratory of the Medical and Experimental Application and Research (ATADEM) in accordance with the Atatürk University’s Local Ethical committee decision (2021/09).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this research article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iskender, H., Dokumacioglu, E., Terim Kapakin, K.A. et al. Effect of Oleanolic acid administration on hepatic AMPK, SIRT-1, IL-6 and NF-κB levels in experimental diabetes. J Diabetes Metab Disord 22, 581–590 (2023). https://doi.org/10.1007/s40200-022-01178-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01178-x